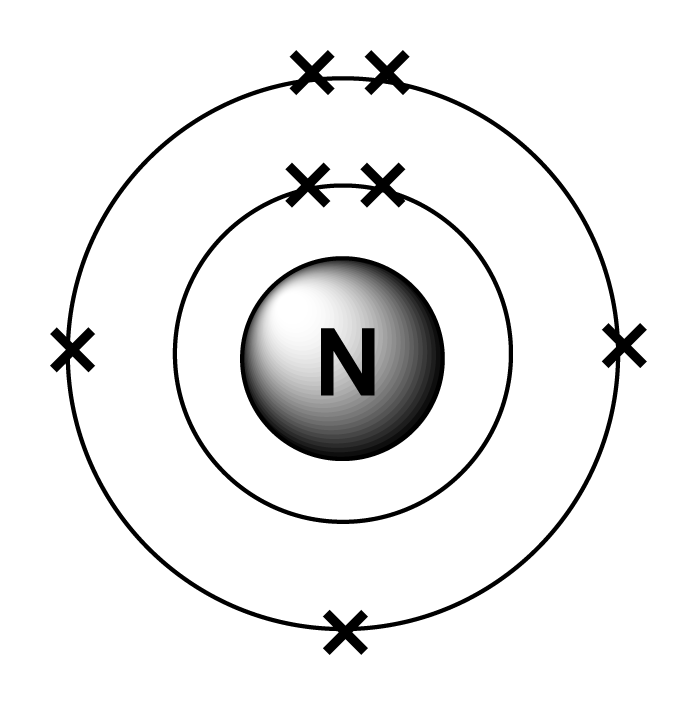
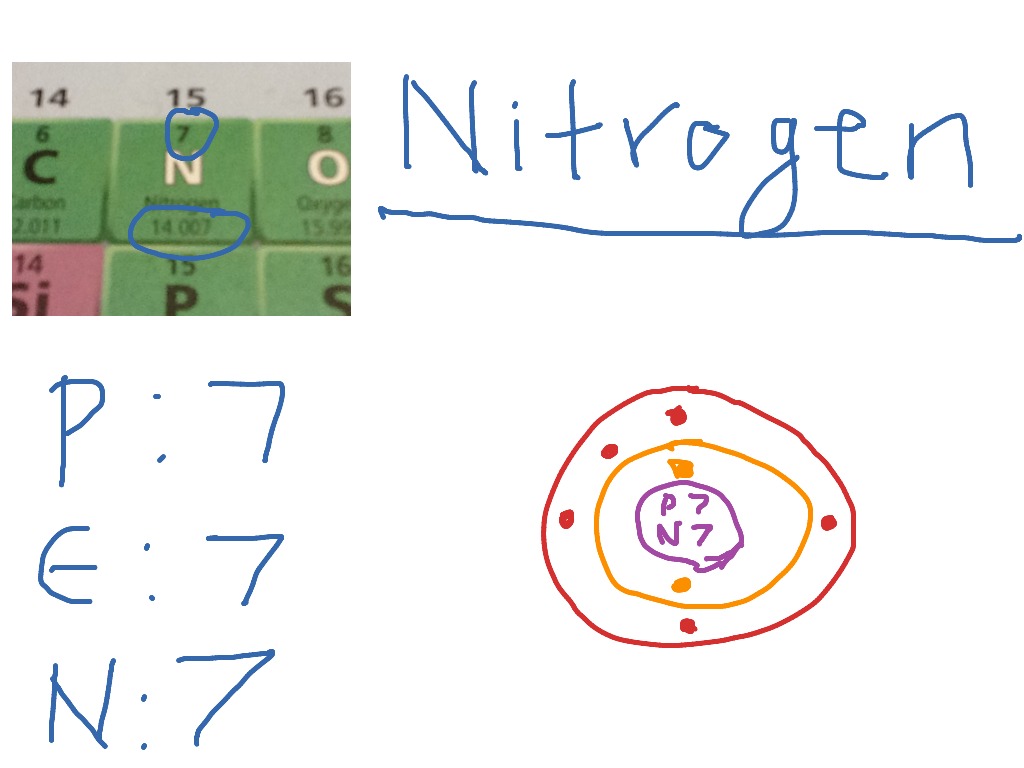
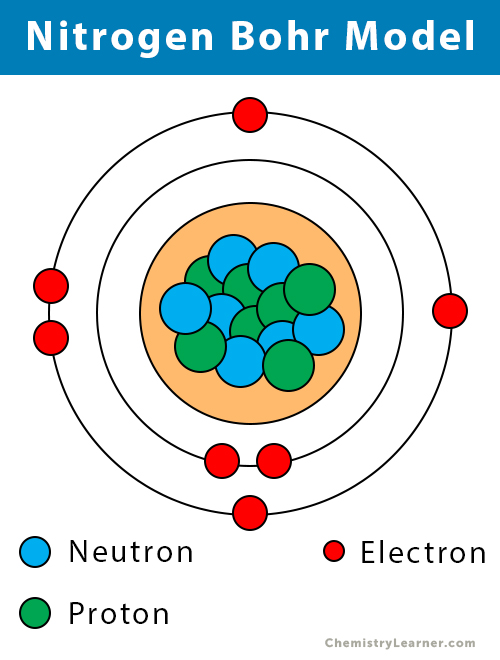
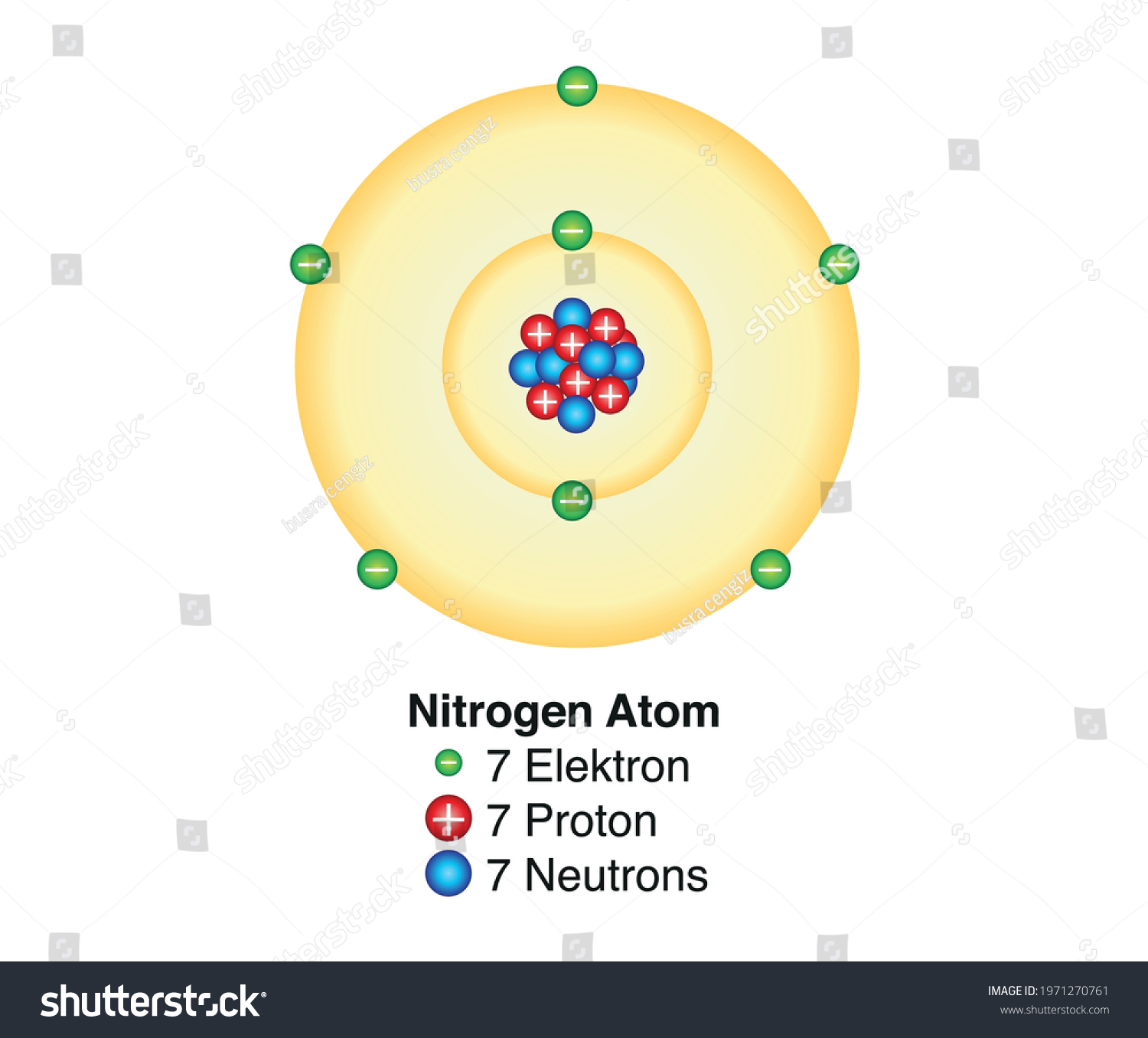
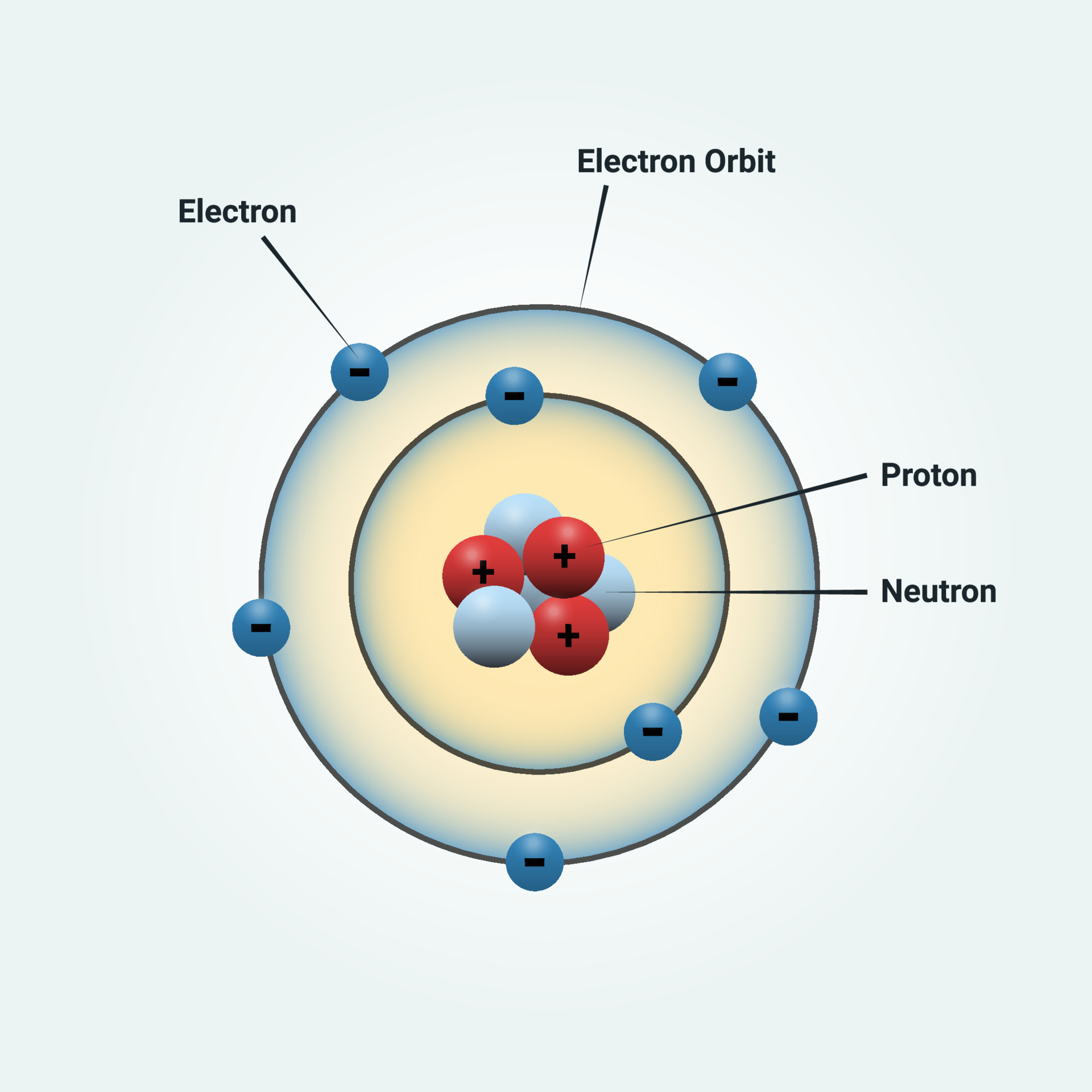
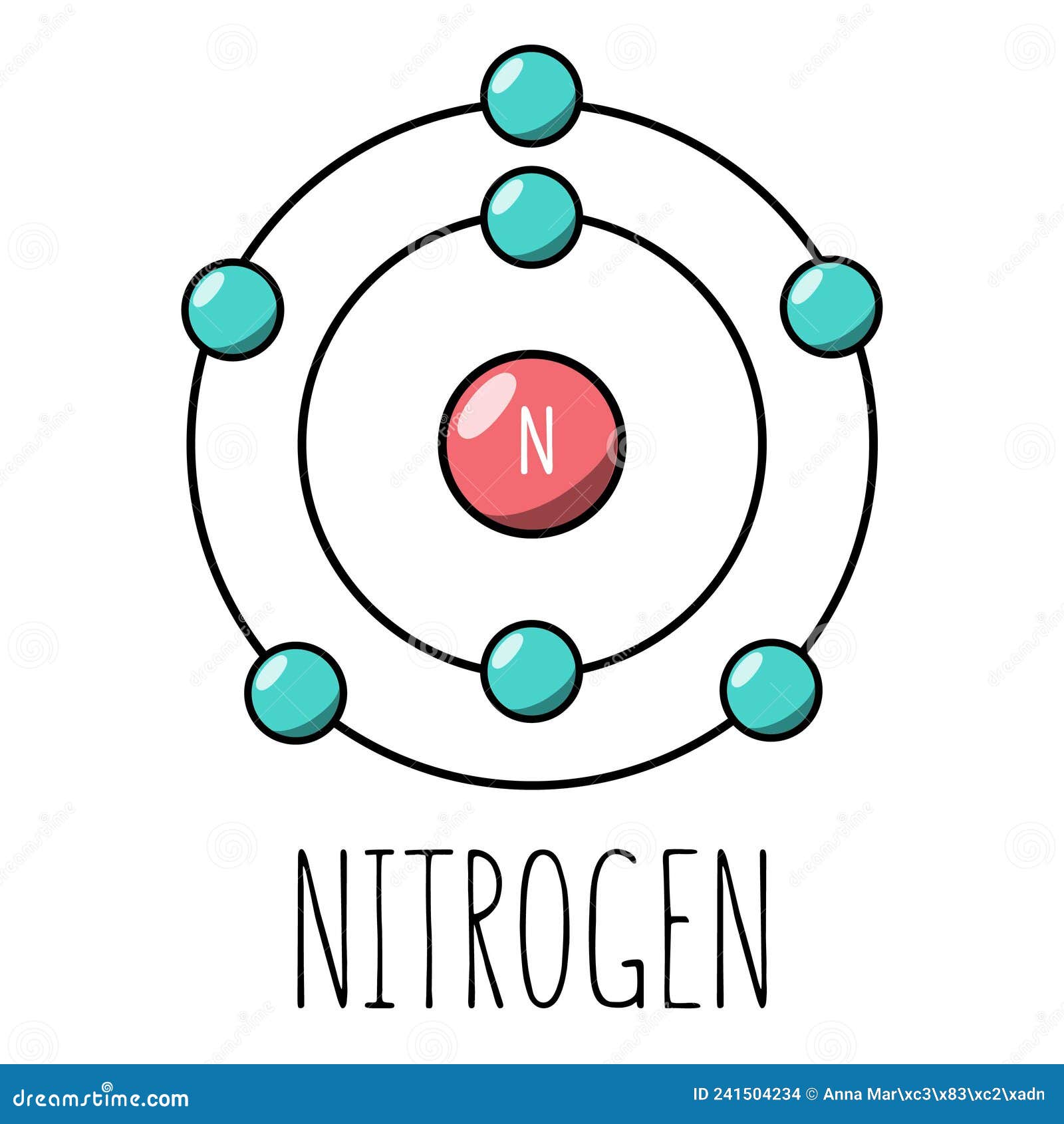
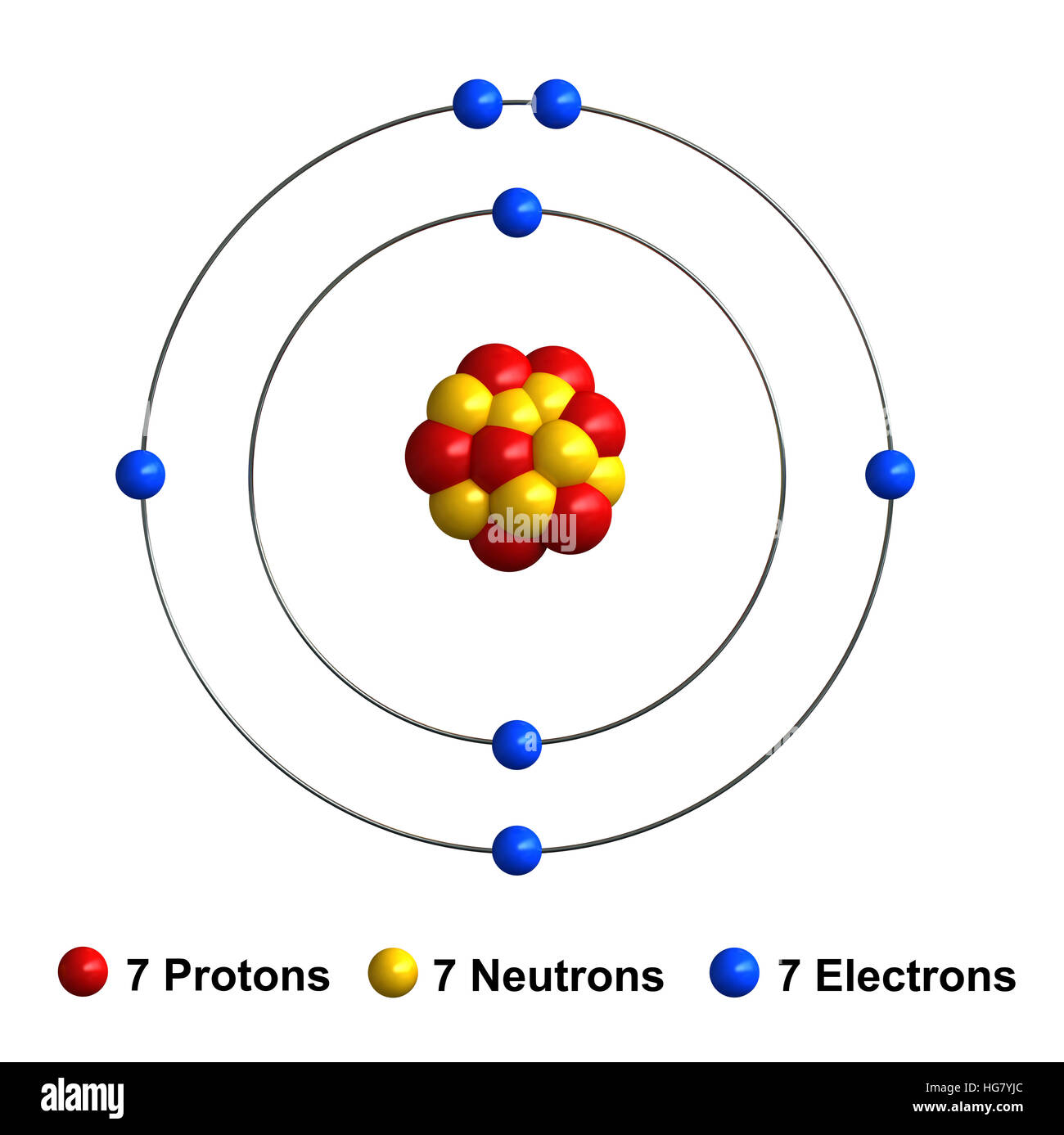
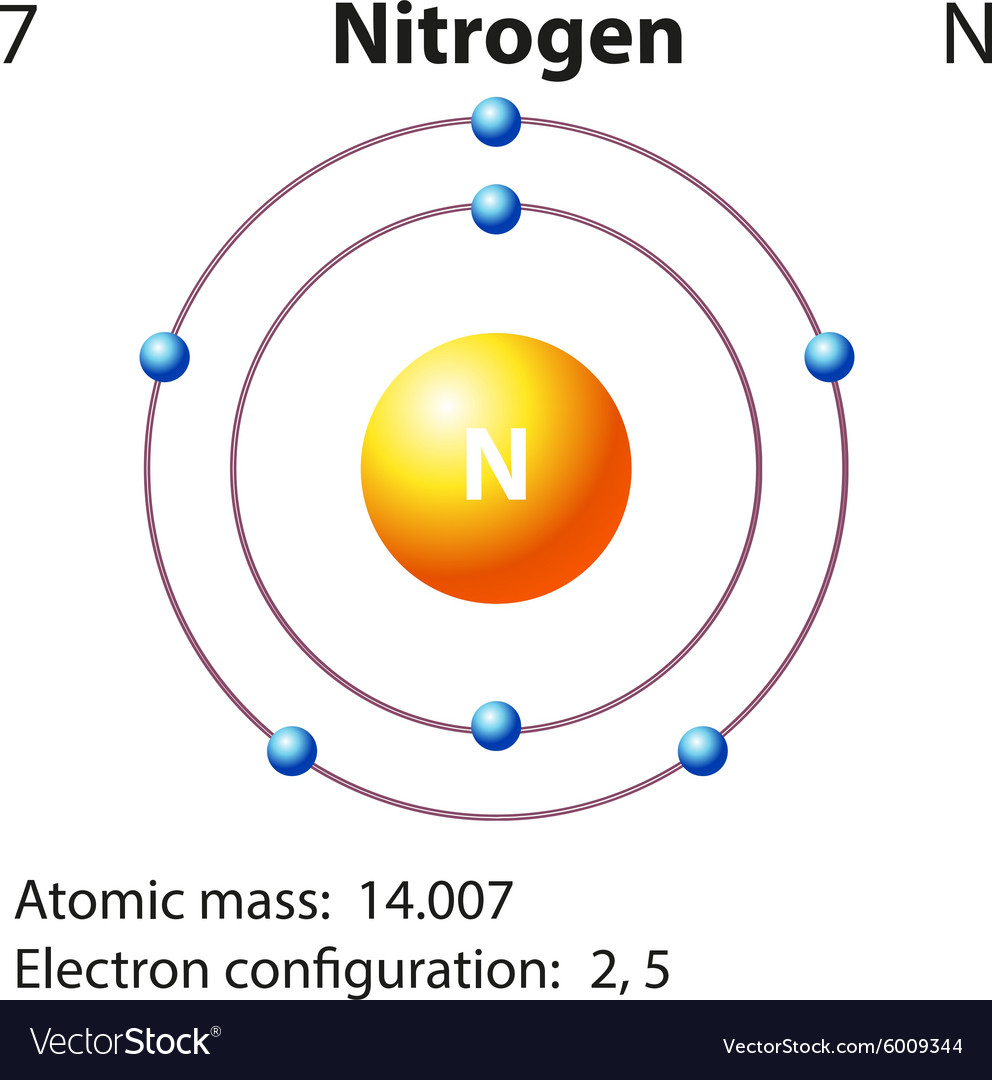
Draw A Nitrogen Atom
Draw A Nitrogen Atom - For the n2 structure use the periodic table to find the total number of valence electrons for. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Web 6 steps to draw the lewis structure of nh3. Web a neutral nitrogen atom has five valence electrons (it is in group 15). If so, follow these instructions to learn where all of the atom's pieces go. The number of protons is the atomic number. Web to write the orbital diagram for the nitrogen atom (n) first we need to write the electron configuration for just n. Web do you need to make a model or a drawing of an atom for science class? A primary (1°) amine has one alkyl (or aryl) group on the. In almost all cases, chemical bonds are formed by interactions of valence electrons in. Here, the given molecule is nh3 (ammonia). In order to draw the lewis structure of nh3, first of all you have to find the total number of valence electrons present in the nh3 molecule. Web nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Web do you need to make a model or a drawing of an atom for science class? The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. From its lewis electron structure, the nitrogen atom in ammonia has one lone pair and. Then play a game to test your ideas! Web to write the orbital diagram for the nitrogen atom (n) first we need to write the electron configuration for just n. The number of protons is the atomic number. A primary (1°) amine has one alkyl (or aryl) group on the. The electronic configuration of nitrogen is 1 s2 2 s2 2. I show you where nitrogen is on the periodic table and how to determine. Web to draw the nitrogen bohr model, represent the 7 protons, 7 neutrons, and 7 electrons. From its lewis electron structure, the nitrogen atom in ammonia has one lone pair and. Web 6 steps to draw the lewis structure of nh3. If so, follow these instructions to learn where all of the atom's pieces go. Web amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom. Nitrogen forms strong bonds because of its. Web the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. Nitrogens atomic number = 7, so there is 7 protons. I show you where nitrogen is on the periodic table and how to determine. Here, the given molecule is nh3 (ammonia). The number of protons is the atomic number. Nitrogen forms strong bonds because of its. A primary (1°) amine has one alkyl (or aryl) group on the. Calculate the total number of valence electrons. Web nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. If so, follow these instructions to learn where all of the atom's pieces go. The number of protons is the atomic number. Web a neutral nitrogen atom has five valence electrons (it is in. Web a neutral nitrogen atom has five valence electrons (it is in group 15). If so, follow these instructions to learn where all of the atom's pieces go. To do that we need to find the number of electrons. A primary (1°) amine has one alkyl (or aryl) group on the. Electrons are the same as protons. Begin by sketching the nucleus, and then draw the two electron shells. Web 6 steps to draw the lewis structure of nh3. Web a neutral nitrogen atom has five valence electrons (it is in group 15). For the n2 structure use the periodic table to find the total number of valence electrons for. Electrons are the same as protons. Web draw a lewis electron dot diagram for an atom or a monatomic ion. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. The electronic configuration of nitrogen is 1 s2 2 s2 2. In almost all cases, chemical bonds are formed by interactions of valence electrons. Nitrogens atomic number = 7, so there is 7 protons. The electronic configuration of nitrogen is 1 s2 2 s2 2. Electrons are the same as protons. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Web to draw the nitrogen bohr model, represent the 7 protons, 7 neutrons, and 7 electrons. I show you where nitrogen is on the periodic table and how to determine. To do that we need to find the number of electrons. Web to draw the nitrogen bohr model, represent the 7 protons, 7 neutrons, and 7 electrons. For the n2 structure use the periodic table to find the total number of valence electrons for. In almost. Web a neutral nitrogen atom has five valence electrons (it is in group 15). Then play a game to test your ideas! Calculate the total number of valence electrons. Nitrogens atomic number = 7, so there is 7 protons. In almost all cases, chemical bonds are formed by interactions of valence electrons in. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. From its lewis electron structure, the nitrogen atom in ammonia has one lone pair and. Here, the given molecule is nh3 (ammonia). Web a neutral nitrogen atom has five valence electrons (it is in group 15). Then play. Web draw a lewis electron dot diagram for an atom or a monatomic ion. To do that we need to find the number of electrons. From its lewis electron structure, the nitrogen atom in ammonia has one lone pair and. Web 6 steps to draw the lewis structure of nh3. In order to draw the lewis structure of nh3, first. Web nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Begin by sketching the nucleus, and then draw the two electron shells. To do that we need to find the number of electrons. Nitrogens atomic number = 7, so there is 7 protons. Web amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom. Web draw a lewis electron dot diagram for an atom or a monatomic ion. A primary (1°) amine has one alkyl (or aryl) group on the. I show you where nitrogen is on the periodic table and how to determine. Then play a game to test your ideas! Web the lewis structure of nitrogen atom can be drawn if one knows the number of valence electrons of nitrogen. From its lewis electron structure, the nitrogen atom in ammonia has one lone pair and. The electronic configuration of nitrogen is 1 s2 2 s2 2. For the n2 structure use the periodic table to find the total number of valence electrons for. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. Nitrogen forms strong bonds because of its. The number of protons is the atomic number.Electron configurations
How to draw a Nitrogen Atom Science ShowMe
Nitrogen Wikipedia
Nitrogen Atom Structure
Basic Model Nitrogen Atom Containing Protons Stock Vector (Royalty Free
Bohr atomic model of a nitrogen atom. vector illustration for science
Nitrogen atom Bohr model stock vector. Illustration of element 241504234
3d render of atom structure of nitrogen isolated over white background
Diagram representation of the element nitrogen Vector Image
Nitrogen, atom model. Chemical element with symbol N and with atomic
Web The Configuration Notation For Nitrogen (N) Provides An Easy Way For Scientists To Write And Communicate How Electrons Are Arranged Around The Nucleus Of The Nitrogen Atom.
Electrons Are The Same As Protons.
Calculate The Total Number Of Valence Electrons.
In Order To Draw The Lewis Structure Of Nh3, First Of All You Have To Find The Total Number Of Valence Electrons Present In The Nh3 Molecule.
Related Post: