Draw All Resonance Structures For The Nitromethane Molecule Ch3No2
Draw All Resonance Structures For The Nitromethane Molecule Ch3No2 - Web chemistry part a draw the lewis structure (including all resonance structures) for nitromethane (ch3no2). Connect the atoms using single bonds. For each resonance structure, assign formal charges to all atoms that. The nitromethane molecule contains a total of. Draw all resonance structures for the nitromethane molecule, ch3no2. Explicitly draw all h atoms. For each resonance structure, assign formal charges to all atoms that have formal. Web identify the locations of lone pairs and possibilities for pi bond rearrangement in the nitromethane molecule to determine the different resonance structures. Web provide the two resonance structures for nitromethane, ch3no2. Draw all the molecules by placing atoms on the grid and. Web identify the locations of lone pairs and possibilities for pi bond rearrangement in the nitromethane molecule to determine the different resonance structures. There are \[5+(3\times6)+1=24\] valence electrons. The lewis structure shows that. The first resonance structure involves the. Web in the case of nitromethane, there are two major resonance structures that contribute to the stability of the molecule. Draw all the molecules by placing atoms on the grid and connecting them. Web in drawing the lewis structure of a compound, we will follow these steps: Give the formal charge of the atoms in each structure (no need to show zero formal charges). Draw all the molecules by placing atoms on the grid and. Nitrogen is then bonded to the carbon and one. Web draw the lewis structure (including resonance structures) for nitromethane (ch_{3}no_{2}). For each resonance structure, assign formal charges to all atoms that have formal. Web the lewis structure for nitromethane (ch3no2) starts with carbon in the center bonded to three hydrogens and the nitrogen atom. 1.draw the lewis structure (including all resonance structures) for nitromethane (ch3no2). We'll form bonds between atoms, and each one of these bonds represents two electrons. Web chemistry part a draw the lewis structure (including all resonance structures) for nitromethane (ch3no2). Include all valence lone pairs in your answer. The nitromethane molecule contains a total of. There are \[5+(3\times6)+1=24\] valence electrons. Explicitly draw all h atoms. Web to draw all resonance structures for the nitromethane molecule, c h x 3 n o x 2 \ce{ch3no2} ch x 3 no x 2 , first let us draw its the lewis structure. There are \[5+(3\times6)+1=24\] valence electrons. Web identify the locations of lone pairs and possibilities for pi bond rearrangement in the nitromethane molecule to determine the different. Nitrogen is then bonded to the carbon and one. Explicitly draw all h atoms. Web provide the two resonance structures for nitromethane, ch3no2. Web identify the locations of lone pairs and possibilities for pi bond rearrangement in the nitromethane molecule to determine the different resonance structures. Web in the case of nitromethane, there are two major resonance structures that contribute. Count the total number of valence electrons in the compound. Web in the case of nitromethane, there are two major resonance structures that contribute to the stability of the molecule. Web in drawing the lewis structure of a compound, we will follow these steps: 1.draw the lewis structure (including all resonance structures) for nitromethane (ch3no2). Connect the atoms using single. Web for the ch3no2 lewis structure, we have a total of 24 valence electrons. Draw all the molecules by placing atoms on the grid and connecting them. For each resonance structure, assign formal charges to all atoms that. 1.draw the lewis structure (including all resonance structures) for nitromethane (ch3no2). Web find the total number of valence electrons present in a. Web the lewis structure for nitromethane (ch3no2) starts with carbon in the center bonded to three hydrogens and the nitrogen atom. Nitrogen is then bonded to the carbon and one. Web identify the locations of lone pairs and possibilities for pi bond rearrangement in the nitromethane molecule to determine the different resonance structures. Explicitly draw all h atoms. Count the. Web in drawing the lewis structure of a compound, we will follow these steps: The lewis structure shows that. The first resonance structure involves the. Draw all the molecules by placing atoms on the grid and. Web a chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. Web draw all resonance structures for the nitromethane molecule, ch3no2. Web identify the locations of lone pairs and possibilities for pi bond rearrangement in the nitromethane molecule to determine the different resonance structures. Web a chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. We'll form bonds between atoms, and. For each resonance structure, assign formal charges to all atoms that have formal. Web find the total number of valence electrons present in a single nitromethane molecule (ch3no2): Nitrogen is then bonded to the carbon and one. Web chemistry part a draw the lewis structure (including all resonance structures) for nitromethane (ch3no2). Draw all the molecules by placing atoms on. The lewis structure shows that. Web in drawing the lewis structure of a compound, we will follow these steps: Draw all resonance structures for the nitromethane molecule, ch3no2. Web in the case of nitromethane, there are two major resonance structures that contribute to the stability of the molecule. Give the formal charge of the atoms in each structure (no need. Explicitly draw all h atoms. Web find the total number of valence electrons present in a single nitromethane molecule (ch3no2): Web draw the lewis structure (including resonance structures) for nitromethane (ch3no2). Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Nitrogen is then bonded to the carbon and one. Connect the atoms using single bonds. The first resonance structure involves the. Web a chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. For each resonance structure, assign formal charges to all atoms that have formal. The lewis structure shows that. Draw all the molecules by placing atoms on the grid and connecting them. Count the total number of valence electrons in the compound. Web identify the locations of lone pairs and possibilities for pi bond rearrangement in the nitromethane molecule to determine the different resonance structures. Include all valence lone pairs in your answer. 1.draw the lewis structure (including all resonance structures) for nitromethane (ch3no2). Web draw the lewis structure (including resonance structures) for nitromethane (ch_{3}no_{2}).CH3NO2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Draw all resonance structures for the nitromethane molecule, Quizlet
SOLVED Draw the Lewis structure (including all resonance structures
SOLVED The structural template for the Lewis structure of nitromethane
Draw all resonance structures for the nitromethane molecule, Quizlet
Ch3No2 Lewis Dot Structure
Draw CH3NO2 (Nitromethane) in Lewis and its conjugate acid YouTube
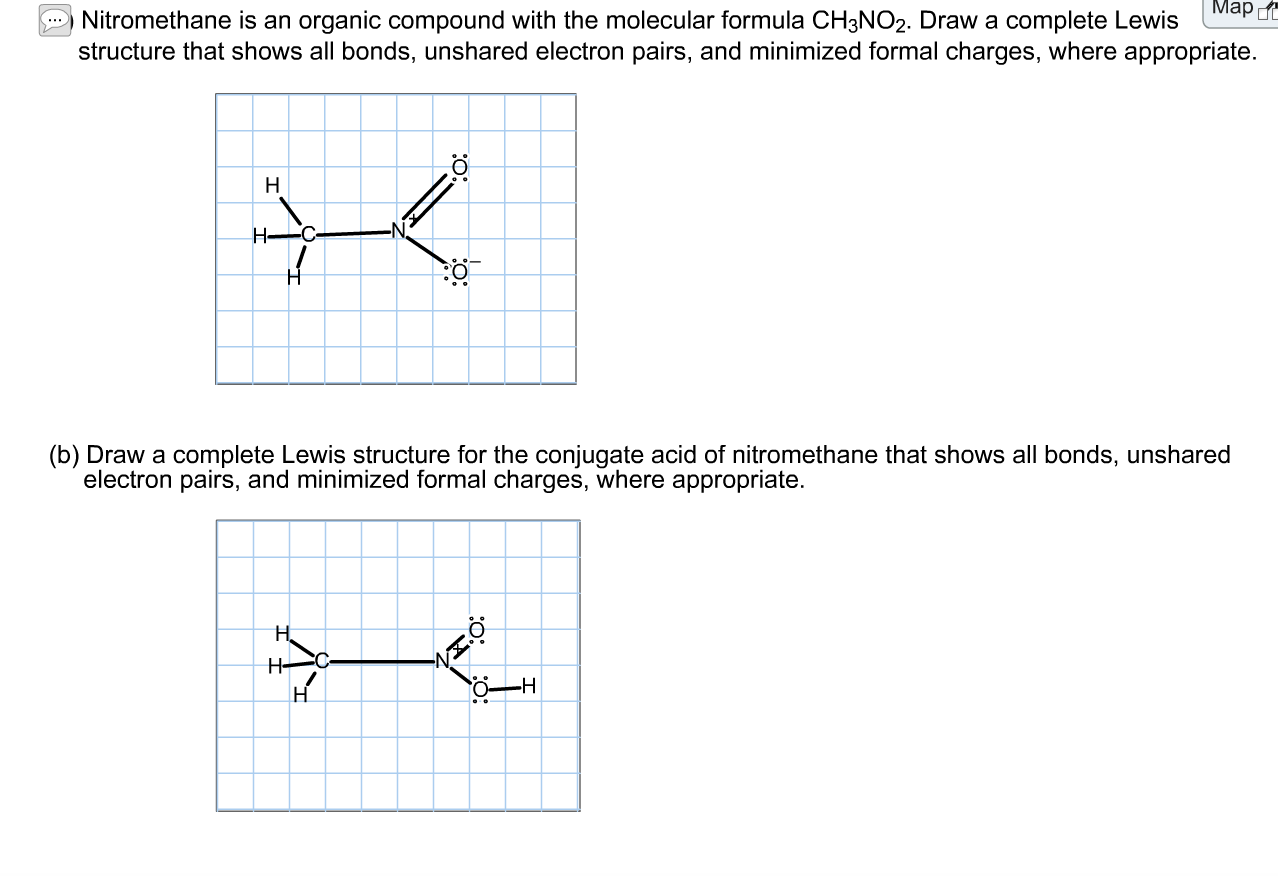
Solved Nitromethane Is An Organic Compound With The Molec...
SOLVED Draw the Lewis structure (including resonance structures) for
Web The Lewis Structure For Nitromethane (Ch3No2) Starts With Carbon In The Center Bonded To Three Hydrogens And The Nitrogen Atom.
Web In The Case Of Nitromethane, There Are Two Major Resonance Structures That Contribute To The Stability Of The Molecule.
Web To Draw All Resonance Structures For The Nitromethane Molecule, C H X 3 N O X 2 \Ce{Ch3No2} Ch X 3 No X 2 , First Let Us Draw Its The Lewis Structure.
For Each Resonance Structure, Assign Formal Charges To All Atoms That.
Related Post: