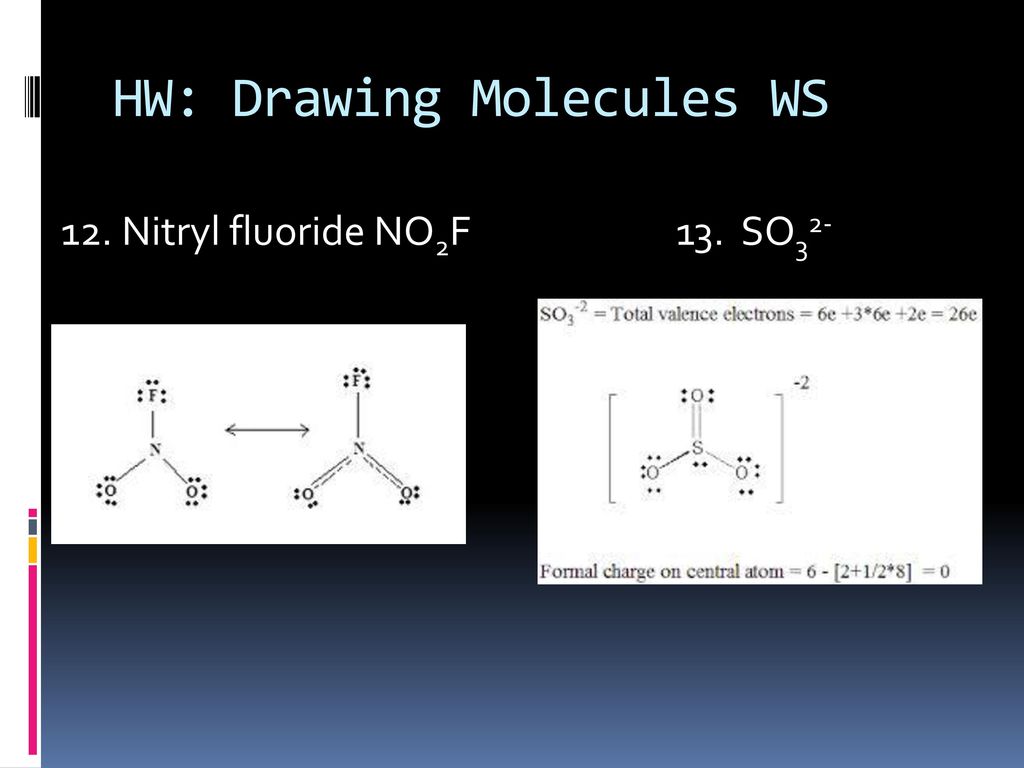
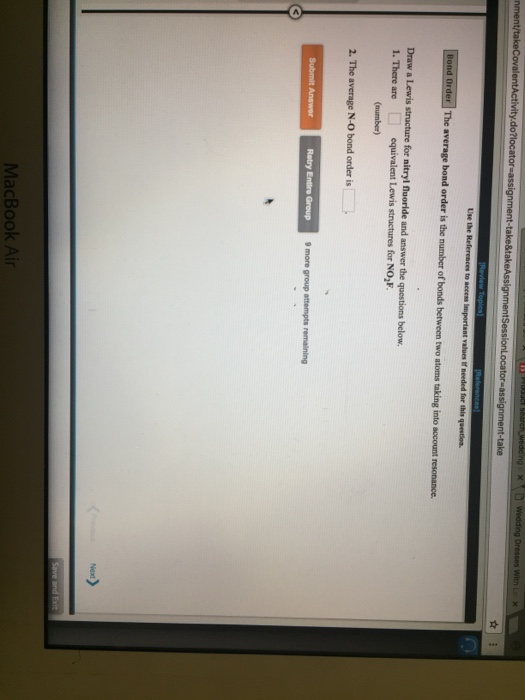
Draw All Resonance Structures For The Nitryl Fluoride Molecule No2F
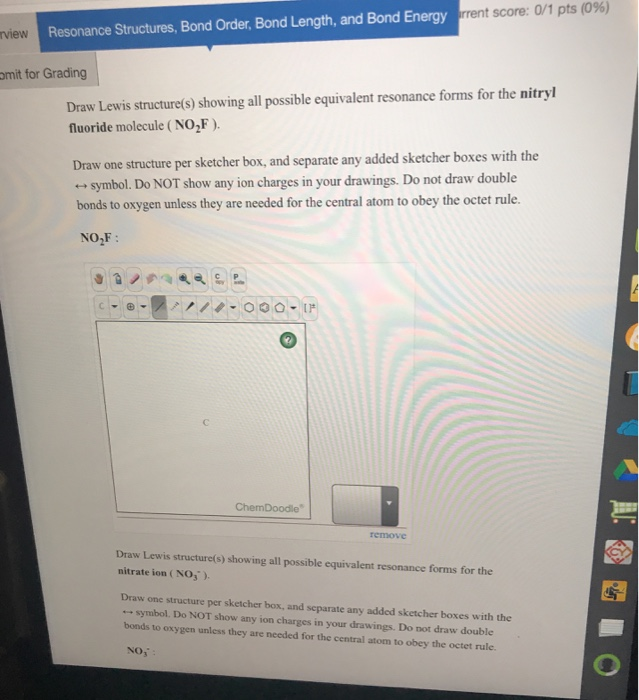
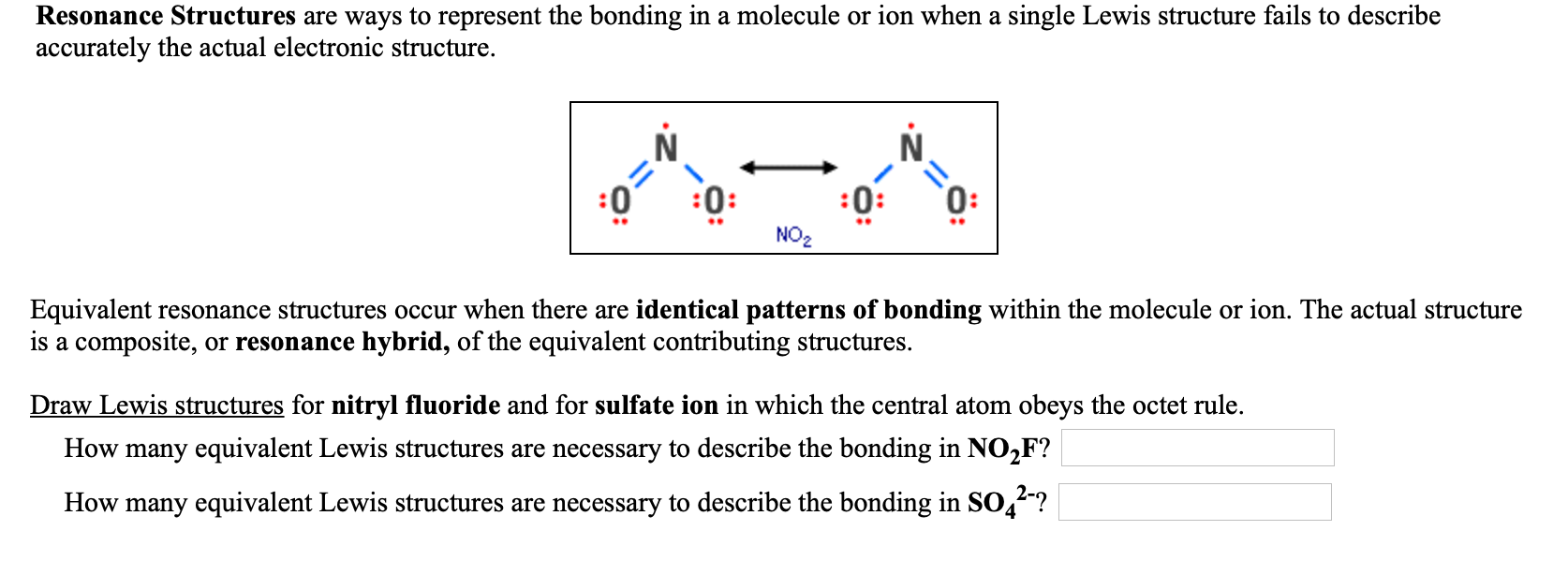
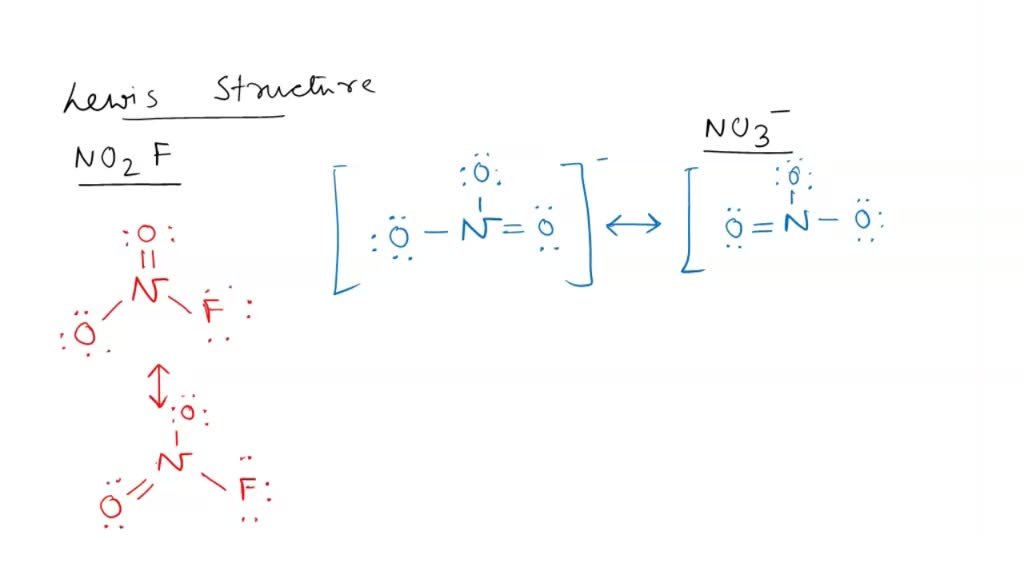
Draw All Resonance Structures For The Nitryl Fluoride Molecule No2F - If there are equivalent resonance structures, draw all of them. Explicitly draw all h atoms. Then draw the 3d molecular structure using vsepr rules: Use getproperty modelinfo or getproperty auxiliaryinfo to inspect. Web this is the in class activity that i use to review the concepts of lewis dot structures, lds, (connectivity, resonance, formal charges, etc.) learned in general chemistry and to. Draw lewis structure (s) showing all possible equivalent resonance forms for the nitryl chloride molecule (no2cl draw one structure per sketcher. Web first draw the lewis dot structure: Web draw all resonance structures for the nitryl fluoride molecule, no2f. Web nitryl fluoride (no2f) is a colorless gas with a pungent odor. • do not include overall ion. Draw one structure per sketcher box, and separate any. Then draw the 3d molecular structure using vsepr rules: • include all valence lone pairs in your answer. If there are equivalent resonance structures, draw all of them. Include all valence lone pairs in your answer. Explicitly draw all h atoms. In the molecule of no₂f, nitrogen has 5 electrons in its valence shell, so it needs 3 electrons to be stable. Explicitly draw all h atoms. Web structure, properties, spectra, suppliers and links for: (c) do not include overall ion. Web first draw the lewis dot structure: Web draw all resonance structures for the nitryl fluoride molecule, no2f. Draw lewis structure (s) showing all possible equivalent resonance forms for the nitryl chloride molecule (no2cl draw one structure per sketcher. Explicitly draw all h atoms. Do not include overall ion. Web the nitryl fluoride molecule (no2f) exhibits resonance, which means it has multiple lewis structures with different electron arrangements. Web draw all resonance structures for the nitryl fluoride molecule, no2f. Include all valence lone pairs in your answer. F | n=o | o. • do not include overall ion. Web draw all resonance structures for the nitryl fluoride molecule, no2f. Use getproperty modelinfo or getproperty auxiliaryinfo to inspect. Web this is the in class activity that i use to review the concepts of lewis dot structures, lds, (connectivity, resonance, formal charges, etc.) learned in general chemistry and to. Web first, let's draw the lewis structure for the nitryl chloride. Web draw lewis structure(s) showing all possible equivalent resonance forms for the nitryl fluoride molecule (no 2 f ). Draw all the resonance structures of the aromatic. Include all valence lone pairs in your answer. Web draw all resonance structures for the nitryl fluoride molecule no2f invites us to explore the intriguing world of molecular bonding, where resonance F |. • do not include overall ion. Web these structures are called resonance structures. F | n=o | o. • include all valence lone pairs in your answer. Web draw all resonance structures for the nitryl fluoride molecule, no₂f. Do not include overall ion. Web draw all resonance structures for the nitryl fluoride molecule, no2f. Web draw all resonance structures for the nitryl fluoride molecule, no2f. In the no 2 f lewis structure, there is one double bond and two single bonds around the. Explicitly draw all h atoms. Web the nitryl fluoride molecule (no2f) exhibits resonance, which means it has multiple lewis structures with different electron arrangements. • do not include overall ion. Do not include overall ion charges. (b) include all valence lone pairs in your answer. Resonance structures are a set of two or more lewis. Explicitly draw all h atoms. F | n=o | o. (b) include all valence lone pairs in your answer. Explicitly draw all h atoms. Web this is the in class activity that i use to review the concepts of lewis dot structures, lds, (connectivity, resonance, formal charges, etc.) learned in general chemistry and to. • do not include overall ion. Use getproperty modelinfo or getproperty auxiliaryinfo to inspect. (c) do not include overall ion. • include all valence lone pairs in your answer. Web draw all resonance structures for the nitryl fluoride molecule, no2f. Draw one structure per sketcher box, and separate any. 1 model in this collection. Explicitly draw all h atoms. Explicitly draw all h atoms. Include all valence lone pairs in your answer. Web draw all resonance structures for the nitryl fluoride molecule, no2f. Do not include overall ion charges. 1 model in this collection. Web draw all resonance structures for the nitryl fluoride molecule, no2f. F | n=o | o. Do not include overall ion charges. In the no 2 f lewis structure, there is one double bond and two single bonds around the. (c) do not include overall ion. • include all valence lone pairs in your answer. Draw lewis structure (s) showing all possible equivalent resonance forms for the nitryl chloride molecule (no2cl draw one structure per sketcher. Web draw lewis structure(s) showing all possible equivalent resonance forms for the nitryl fluoride molecule (no 2 f ). • do not include overall ion. Web draw all resonance structures for the nitryl fluoride molecule no2f invites us to explore the intriguing world of molecular bonding, where resonance • include all valence lone pairs in your answer. Then draw the 3d molecular structure using vsepr rules: Do not include overall ion charges. 1 model in this collection. It is a powerful oxidizing agent and is used in the production of rocket propellants and explosives. Explicitly draw all h atoms. Include all valence lone pairs in your answer. Web no 2 f (nitryl fluoride) has one nitrogen atom, two oxygen atoms, and one fluorine atom. In the no 2 f lewis structure, there is one double bond and two single bonds around the. Web resonance structures for this molecule can be drawn by moving the double bond between nitrogen and oxygen. Draw all resonance structures for the nitryl fluoride molecule, no2 f. Web draw all resonance structures for the nitryl fluoride molecule, no2f. Web this is the in class activity that i use to review the concepts of lewis dot structures, lds, (connectivity, resonance, formal charges, etc.) learned in general chemistry and to.Draw All Resonance Structures for the Nitryl Fluoride Molecule No2f
No2f Resonance Structures
Draw All Resonance Structures for the Nitryl Fluoride Molecule No2f
Draw All Resonance Structures for the Nitryl Fluoride Molecule No2f
Draw All Resonance Structures for the Nitryl Fluoride Molecule No2f
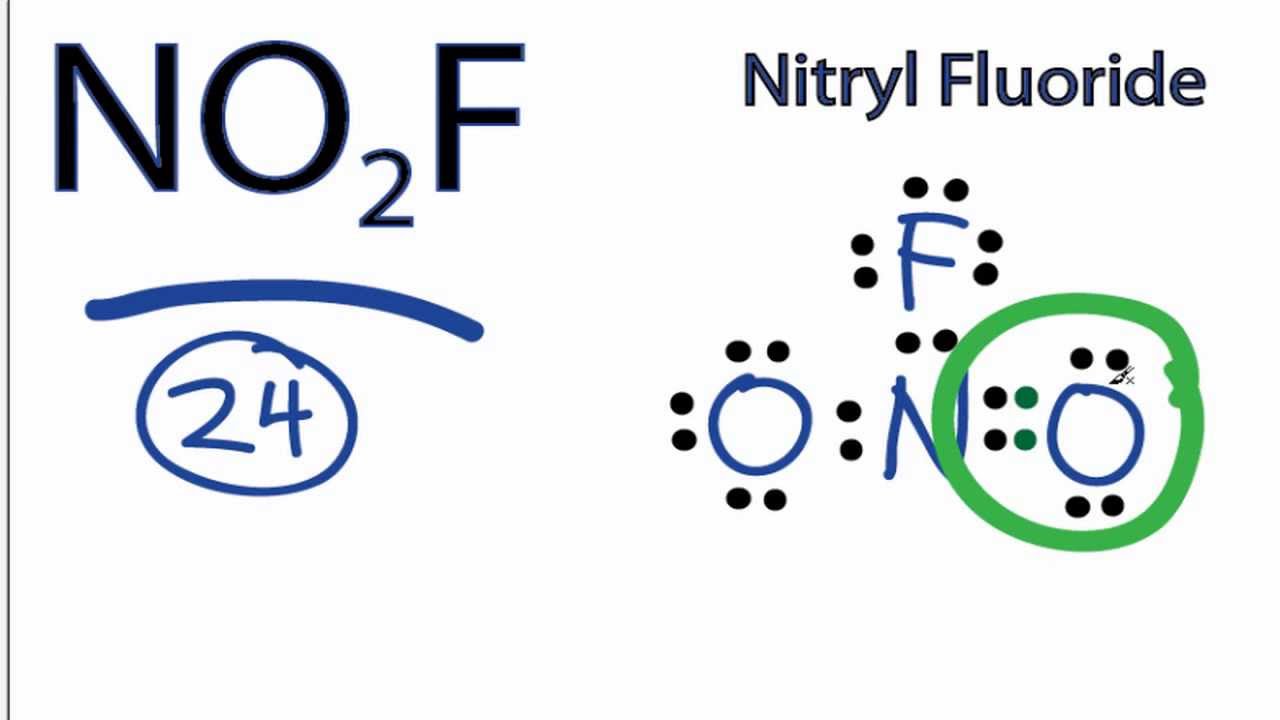
NO2F Lewis Structure How to Draw the Lewis Structure for NO2F YouTube
NO2F Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Draw All Resonance Structures for the Nitryl Fluoride Molecule No2f
Draw All Resonance Structures for the Nitryl Fluoride Molecule No2f
No2f Resonance Structures
(A) Explicitly Draw All H Atoms.
Explicitly Draw All H Atoms.
Web Nitryl Fluoride (No2F) Is A Colorless Gas With A Pungent Odor.
Use Getproperty Modelinfo Or Getproperty Auxiliaryinfo To Inspect.
Related Post: