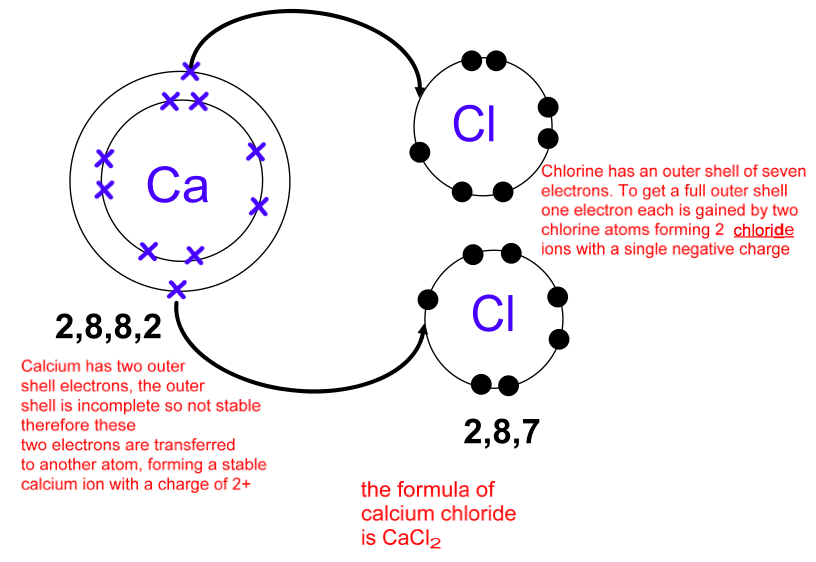
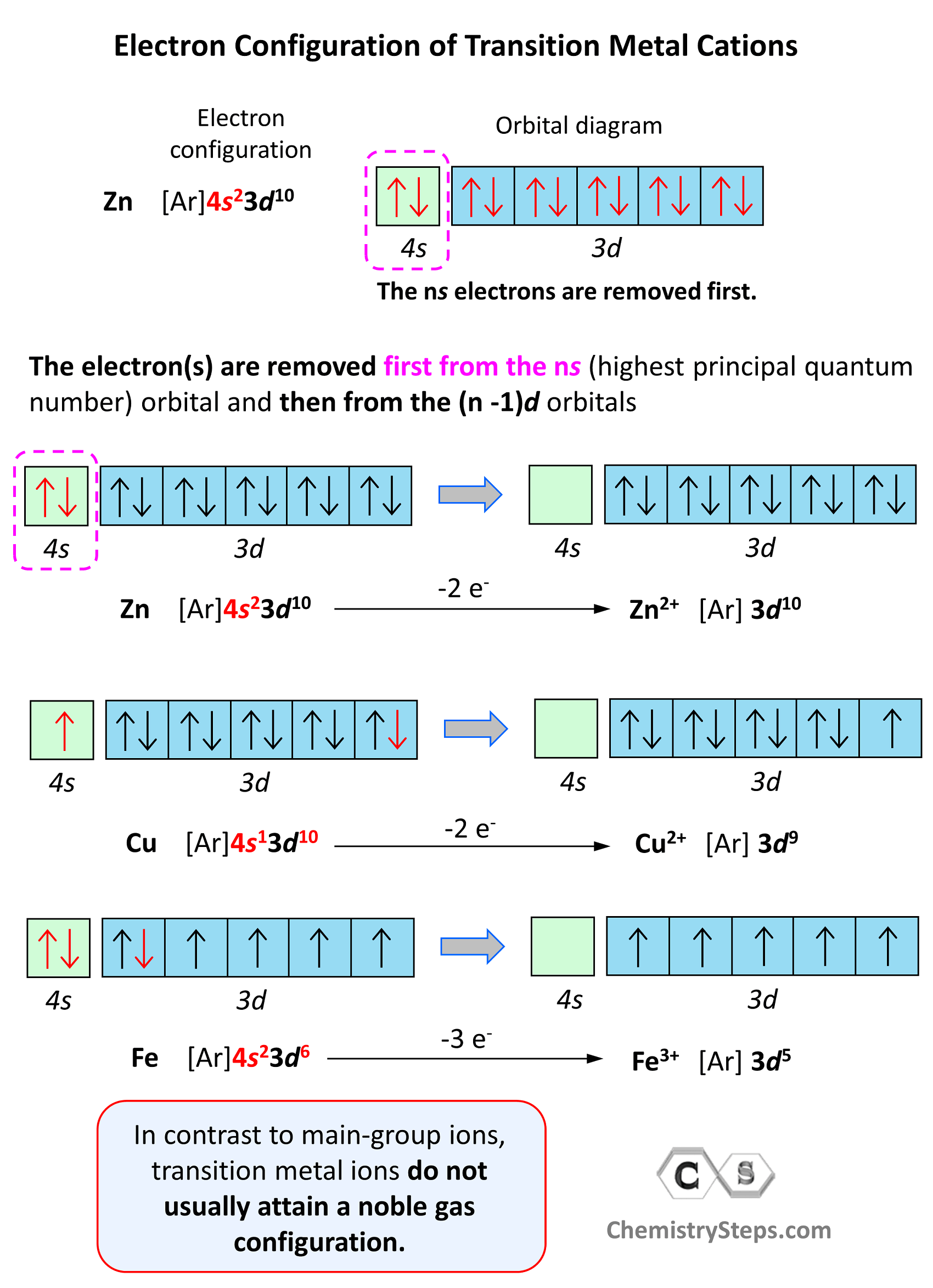
Draw An Outer Electron Box Diagram For A Cation
Draw An Outer Electron Box Diagram For A Cation - Web the outer electron box diagram represents the electrons in the outermost energy levels (4d and 5s in this case). $\boxed{\begin{array}{c|c|c|c|c|c|c} \text{3s} & \text{3p} & \text{3p} & \text{3p} & \text{3d} & \text{3d} & \text{3d} \\ \hline. Your solution’s ready to go! Draw the electron configuration for cation. Represent the 4d orbital with 5 boxes, each box representing an electron. Distinguish between outer energy level (valence) electrons and core electrons. Ru 2 + draw an outer electron box diagram for cation. Which transition metal could this be? Web give the electron configuration for an atom using bohr’s model, box orbital diagrams, and quantum mechanical notation. Web draw an outer electron box diagram for a tc cation. Web the outer electron box diagram for the v³⁺ cation is: Lo students in this question. Web bohr diagrams indicate how many electrons fill each principal shell. Connect the electron pairs in the [xe]5d6. 4+ draw an outer electron box diagram for a nb cation. Web here’s how to approach this question. Web draw an outer electron box diagram for a tc cation. Web draw an outer electron box diagram for a mn4+ cation. Represent the 4d orbital with 5 boxes, each box representing an electron. Web drawing an outer electron box diagram for a cation helps students understand the concept of valence electrons by visually representing the arrangement of electrons in the. Identify and explain exceptions to predicted electron configurations for atoms and ions. Which transition metal could this be? Web here’s how to approach this question. Web the outer electron box diagram represents the electrons in the outermost energy levels (4d and 5s in this case). Generally, the electronic configuration of d block elements is ( n − 1) d 1 − 10 n s 1 − 2 (. Determine the electron configuration for the tc3+ cation, taking into account the removal of electrons due to the formation of the cation. Web draw a dot and cross diagram to show the electron structure of the compound tetrachloromethane (only the outer electrons need to be shown). Your solution’s ready to go! Connect the electron pairs in the [xe]5d6. Represent the 4d orbital with 5 boxes, each box representing an electron. In this case, the cation has an outer electron configuration of [xe]5d6. Web draw an outer electron box diagram for a mn4+ cation. Generally, the electronic configuration of d block elements is ( n − 1) d 1 − 10 n s 1 − 2 (. Web draw a dot and cross diagram to show the electron structure of the. Web give the electron configuration for an atom using bohr’s model, box orbital diagrams, and quantum mechanical notation. Your solution’s ready to go! Since tc2+ has a 2+ charge, we remove 2 electrons from the 4d orbital. Web since the electrons in the outermost energy level determine the chemical properties of an element, the outer electron box diagram for a. Draw the electron configuration for cation. Web bohr diagrams indicate how many electrons fill each principal shell. Represent the 4d orbital with 5 boxes, each box representing an electron. Web give the electron configuration for an atom using bohr’s model, box orbital diagrams, and quantum mechanical notation. Web draw an outer electron box diagram for a tc cation. Connect the electron pairs in the [xe]5d6. Draw the electron configuration for cation. Ru 2 + draw an outer electron box diagram for cation. Generally, the electronic configuration of d block elements is ( n − 1) d 1 − 10 n s 1 − 2 (. Web here’s how to approach this question. Lo students in this question. Draw an outer electron box diagram for a n b 3 + cation. Web convert from orbital representation diagrams to electron configuration codes. Determine the electron configuration for the tc3+ cation, taking into account the removal of electrons due to the formation of the cation. Since tc2+ has a 2+ charge, we remove 2 electrons. Determine the electron configuration for the tc3+ cation, taking into account the removal of electrons due to the formation of the cation. Web bohr diagrams indicate how many electrons fill each principal shell. Web draw an outer electron box diagram for a tc cation. Electron configurations and box orbital diagrams for ions. We practice doing both an anion and cation. A + 3 cation of a certain transition metal has two electrons in its outermost d subshell. Web draw an outer electron box diagram for a mn4+ cation. We will draw boxes for each orbital in the 4d and 5s subshells. Web the outer electron box diagram represents the electrons in the outermost energy levels (4d and 5s in this. Which transition metal could this be? Electron configurations and box orbital diagrams for ions. $\boxed{\begin{array}{c|c|c|c|c|c|c} \text{3s} & \text{3p} & \text{3p} & \text{3p} & \text{3d} & \text{3d} & \text{3d} \\ \hline. Lo students in this question. Web drawing the outer electron box diagram of a transition metal cation draw an outer electron box diagram for a zrcation. Distinguish between outer energy level (valence) electrons and core electrons. A + 3 cation of a certain transition metal has two electrons in its outermost d subshell. Generally, the electronic configuration of d block elements is ( n − 1) d 1 − 10 n s 1 − 2 (. We will draw boxes for each orbital in the 4d. Web the outer electron box diagram for the v³⁺ cation is: Your solution’s ready to go! Determine the electron configuration for the tc3+ cation, taking into account the removal of electrons due to the formation of the cation. Identify and explain exceptions to predicted electron configurations for atoms and ions. This video also has a tip on how to tell. A + 3 cation of a certain transition metal has two electrons in its outermost d subshell. We will draw boxes for each orbital in the 4d and 5s subshells. Lo students in this question. Your solution’s ready to go! Web draw an outer electron box diagram for a tc cation. Web convert from orbital representation diagrams to electron configuration codes. Represent the 4d orbital with 5 boxes, each box representing an electron. Distinguish between outer energy level (valence) electrons and core electrons. Web since the electrons in the outermost energy level determine the chemical properties of an element, the outer electron box diagram for a cation can help us understand how the. Which transition metal could this be? Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. 4+ draw an outer electron box diagram for a nb cation. Since tc2+ has a 2+ charge, we remove 2 electrons from the 4d orbital. Web draw a dot and cross diagram to show the electron structure of the compound tetrachloromethane (only the outer electrons need to be shown). $\boxed{\begin{array}{c|c|c|c|c|c|c} \text{3s} & \text{3p} & \text{3p} & \text{3p} & \text{3d} & \text{3d} & \text{3d} \\ \hline. In this case, the cation has an outer electron configuration of [xe]5d6.Outer Electron Box Diagram For A Cation Simplify And Visualize
ALevel Chemistry 1.6.1c draw electron configuration diagrams of
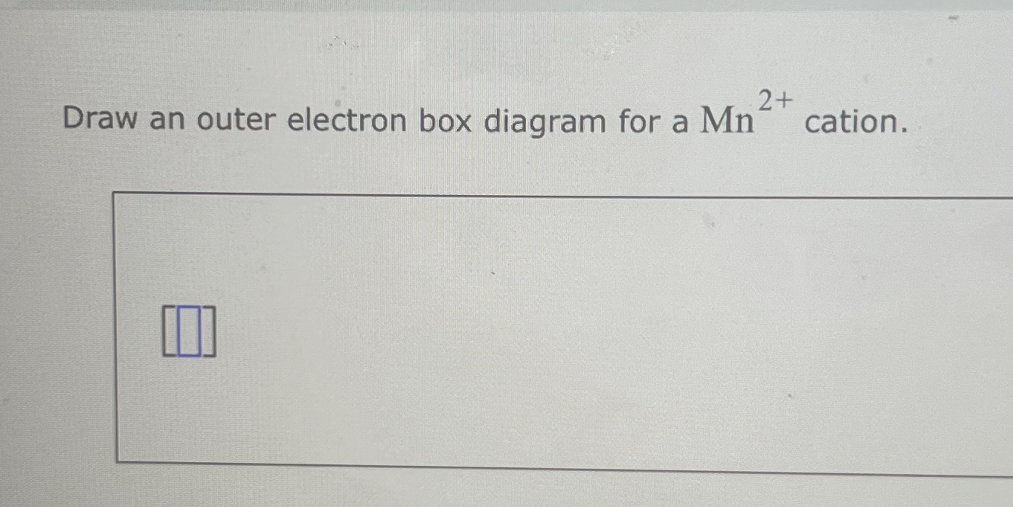
SOLVED Draw an outer electron box diagram for a Mn^2+ cation.
ALevel Chemistry 1.6.1c draw electron configuration diagrams of
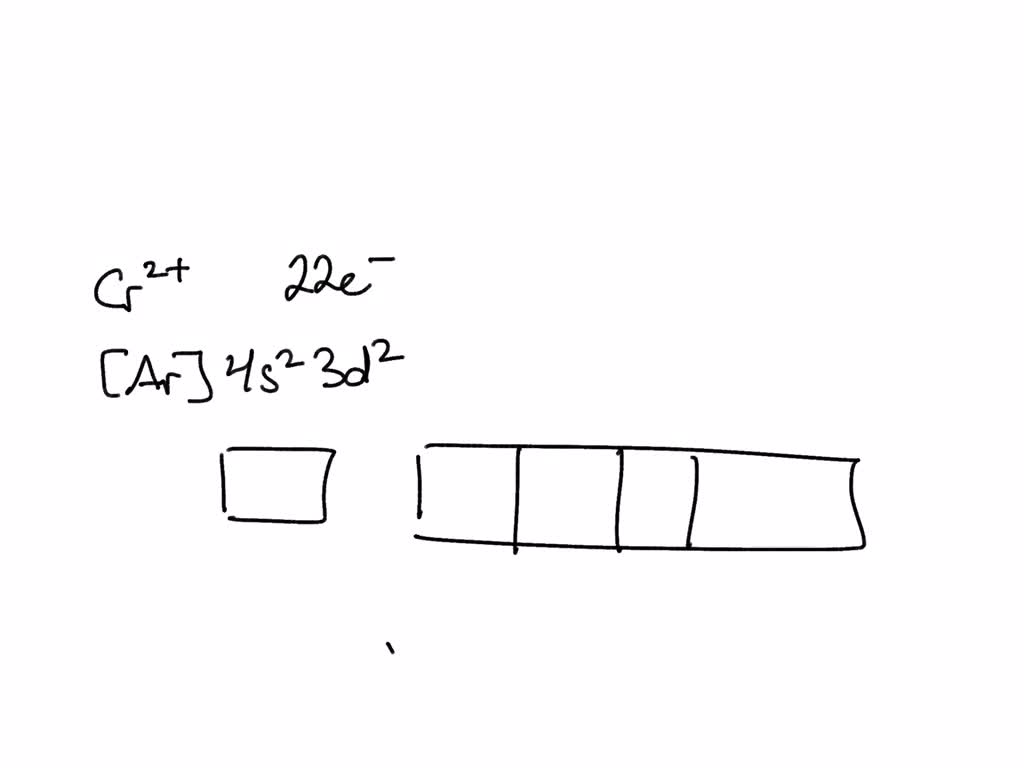
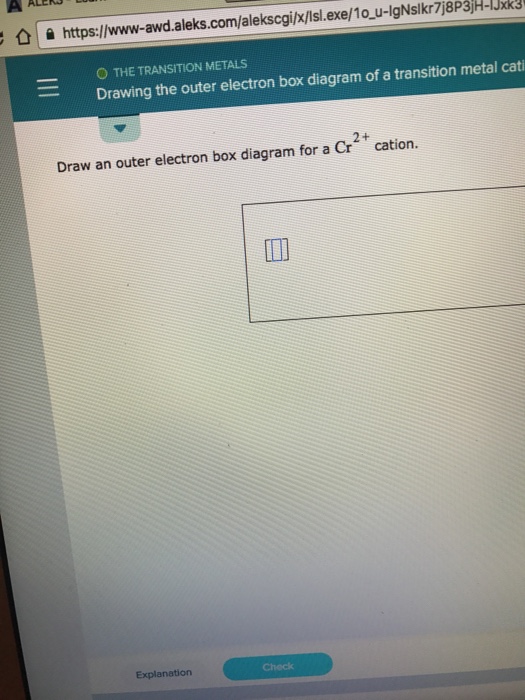
SOLVED Draw an outer electron box diagram for Cr2 cation;
Solved Draw an outer electron box diagram for a Mo4+ cation.
Draw An Outer Electron Box Diagram For A Cr^2+ Cat...
SOLVED '3 Draw an outer electron box diagram for Tc cation.'
Electron Configurations of Ions Chemistry Steps
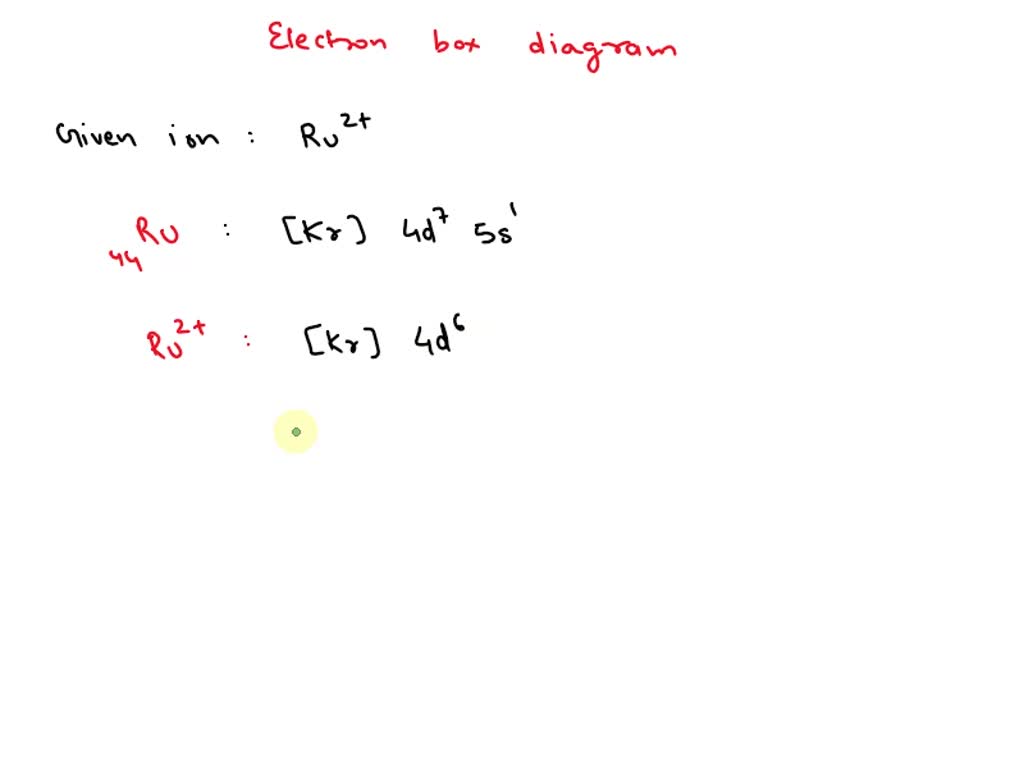
SOLVED Ru 2 + Draw an outer electron box diagram for cation
Draw An Outer Electron Box Diagram For A N B 3 + Cation.
Web Draw An Outer Electron Box Diagram For A Mn4+ Cation.
Generally, The Electronic Configuration Of D Block Elements Is ( N − 1) D 1 − 10 N S 1 − 2 (.
Ru 2 + Draw An Outer Electron Box Diagram For Cation.
Related Post: