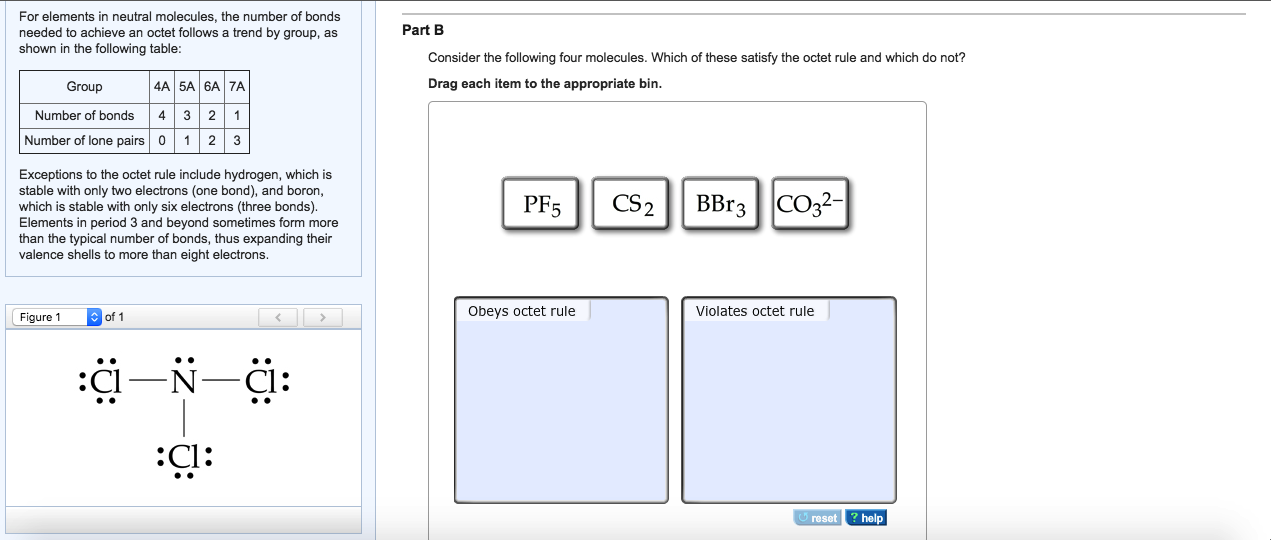
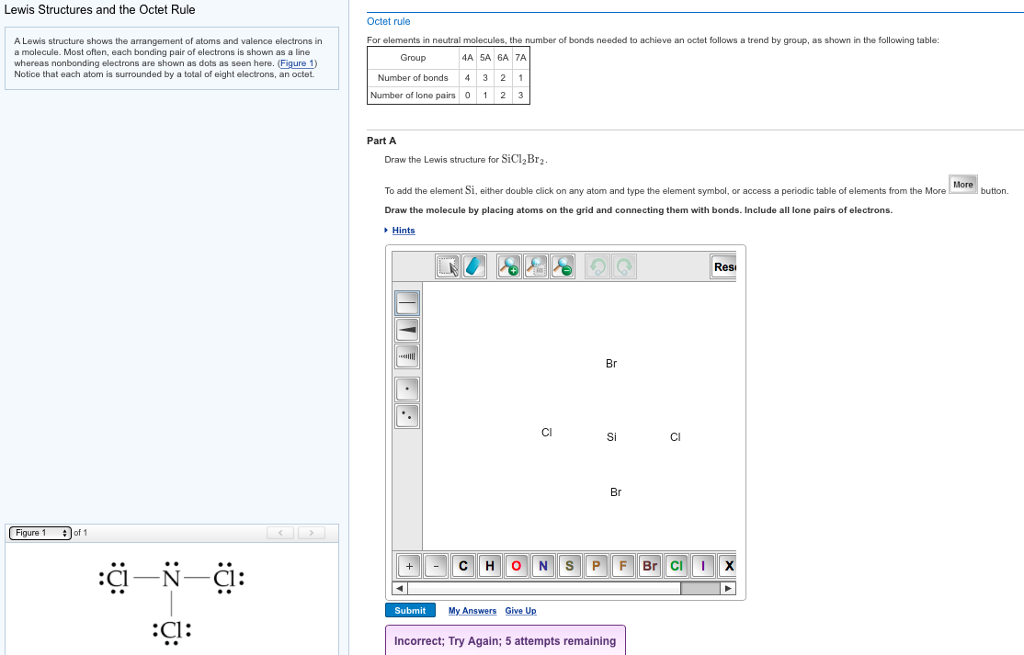
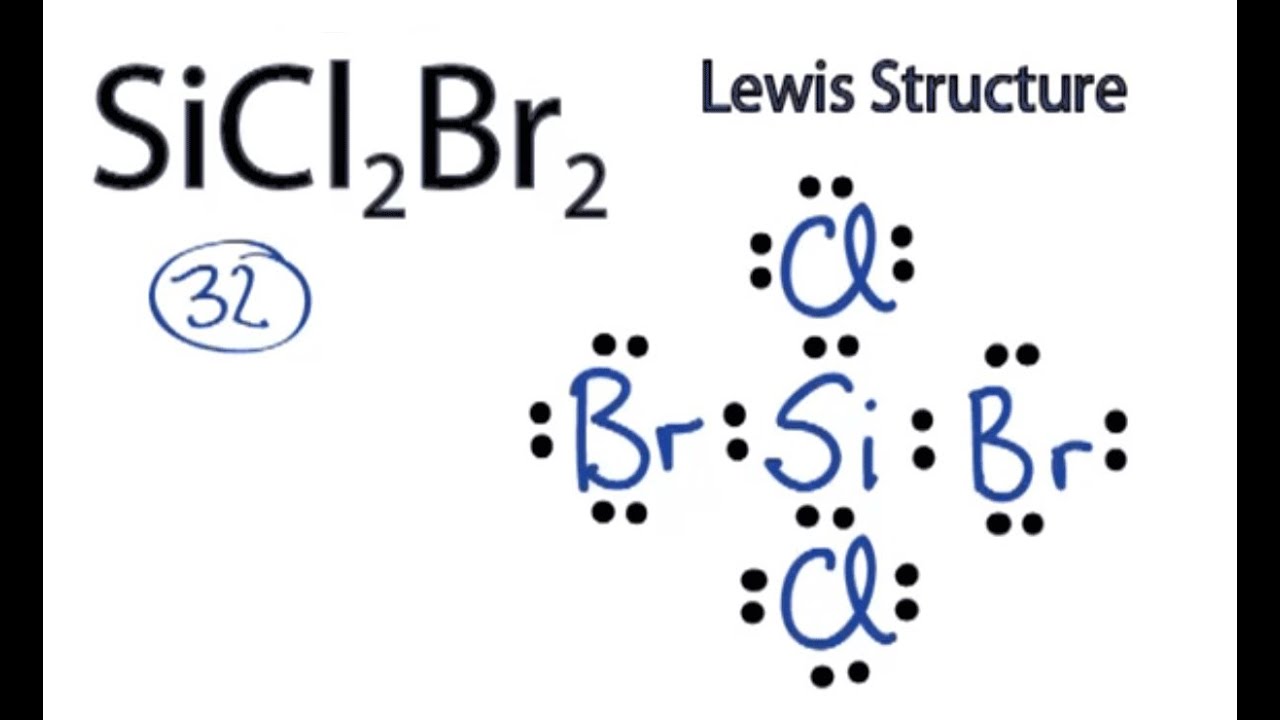Draw Lewis Structure For Sicl2Br2
Draw Lewis Structure For Sicl2Br2 - Web this video shows you how to draw the lewis dot structure for sicl2br2. Include all lone pairs of electrons. Web to draw the lewis structure for sicl2br2, we need to follow a process: Draw the molecule by placing atoms on the grid and connecting them with bonds. Web sicl2br2 is a chemical formula for dibromo dichloro silane. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web the lewis structure for the molecule sicl2br2 is drawn by following a set of guidelines. Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has 7 valence. Lewis structures of molecular compounds. Lewis structures of molecular compounds. Web in this article, “sicl2br2 lewis structure”, lewis structure drawing, hybridization, shape, formal charge calculation with some detailed explanations are. The lewis structure for sicl2br2 (silicon tetrachloride dibromide) can be. Your solution’s ready to go! Web lewis structures are very similar to electron dot diagrams except for the fact that shared electrons between atoms are shown as lines. Web the lewis structure for the molecule sicl2br2 is drawn by following a set of guidelines. Each halogen atom forms a. Web this video shows you how to draw the lewis dot structure for sicl2br2. Web how do you draw a lewis structure for sicl_2br_2? Determine the total number of valence electrons in the molecule. It is no longer used for this purpose because of the formation of the toxic gas phosgene, cl 2 co. Web drawing lewis structures for molecules with one central atom: To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the. Determine the total number of valence electrons: Web to properly draw the sicl 2 br 2 lewis structure, follow these steps: Web by using the following steps, you can easily draw the lewis structure of sicl 2 br 2. Web in this article, “sicl2br2 lewis structure”, lewis structure drawing, hybridization, shape, formal charge calculation with some detailed explanations are. The following procedure will give you the correct lewis structure for any. The lewis structure for sicl2br2 (silicon tetrachloride dibromide) can be. Firstly, silicon (si) is the least electronegative and is chosen as the central. Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has 7 valence. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis. Web carbon tetrachloride was formerly used in fire extinguishers for electrical fires. Draw the molecule by placing atoms on the grid and connecting them with. Web the lewis structure for sicl2br2 is where silicon is in the center, surrounded by two chlorine atoms and two bromine atoms. Lone pairs of electrons themselves are. Web the lewis structure for sicl2br2 can be drawn as: Firstly, silicon (si) is the least electronegative and is chosen as the central. Web sicl2br2 is a chemical formula for dibromo dichloro. Web sicl2br2 is a chemical formula for dibromo dichloro silane. Web this video shows you how to draw the lewis dot structure for sicl2br2. It also provides the molecular geometry and bond angle for sicl2br2 as well. Lewis structures of molecular compounds. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3. Determine the total number of valence electrons: Web lewis structures are very similar to electron dot diagrams except for the fact that shared electrons between atoms are shown as lines. Web by using the following steps, you can easily draw the lewis structure of sicl 2 br 2. Each halogen atom forms a. To add the element si, either double. Web how do you draw a lewis structure for sicl_2br_2? Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has 7 valence. Your solution’s ready to go! Each halogen atom forms a. Draw the molecule by placing atoms on the grid and connecting them with bonds. Lewis structures of molecular compounds. It is no longer used for this purpose because of the formation of the toxic gas phosgene, cl 2 co. Web how do you draw a lewis structure for sicl_2br_2? Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has 7 valence. Web carbon tetrachloride was formerly used in. Firstly, silicon (si) is the least electronegative and is chosen as the central. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate. The following procedure will give you the correct lewis structure for any. Web part a draw the lewis structure for sicl2br2. Web the lewis structure for sicl2br2 is where silicon is in the center,. The following procedure will give you the correct lewis structure for any. Web to properly draw the sicl 2 br 2 lewis structure, follow these steps: #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate. Web sicl2br2 is a chemical formula for dibromo dichloro silane. Web draw the lewis structure for sicl2br2. Web by using the following steps, you can easily draw the lewis structure of sicl 2 br 2. Web to draw the lewis structure for sicl2br2, we need to follow a process: #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3. Here’s the best way to. Web this widget gets the lewis. Web how do you draw a lewis structure for sicl_2br_2? Draw the molecule by placing atoms on the grid and connecting them with bonds. Web this video shows you how to draw the lewis dot structure for sicl2br2. Web lewis structures are very similar to electron dot diagrams except for the fact that shared electrons between atoms are shown as. Here’s the best way to. It also provides the molecular geometry and bond angle for sicl2br2 as well. Firstly, silicon (si) is the least electronegative and is chosen as the central. Draw the molecule by placing atoms on the grid and connecting them with bonds. The following procedure will give you the correct lewis structure for any. Include all lone pairs of electrons. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis. Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has 7 valence. Web the lewis structure for sicl2br2 is where silicon is in the center, surrounded by two chlorine atoms and two bromine atoms. Each halogen atom forms a. Web sicl2br2 is a chemical formula for dibromo dichloro silane. Determine the total number of valence electrons: Web drawing lewis structures for molecules with one central atom: Web lewis structures are very similar to electron dot diagrams except for the fact that shared electrons between atoms are shown as lines. Web this widget gets the lewis structure of chemical compounds. It is no longer used for this purpose because of the formation of the toxic gas phosgene, cl 2 co.Draw the Lewis structure for SiCl2Br2. HomeworkLib
SiCl2Br2 Lewis Structure, Geometry, Hybridization, and Polarity
Sicl2br2 Lewis Structure How To Draw The Lewis Structure
Draw The Lewis Structure For Sicl2br2
lewis structure for sicl2br2
Lewis Structure of SiCl2Br2 YouTube
lewis structure for sicl2br2
SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
lewis structure for sicl2br2
Web To Properly Draw The Sicl 2 Br 2 Lewis Structure, Follow These Steps:
To Add The Element Si, Either Double Click On Any Atom And Type The Element Symbol, Or Access A Periodic Table Of Elements From The.
Web This Video Shows You How To Draw The Lewis Dot Structure For Sicl2Br2.
Your Solution’s Ready To Go!
Related Post: