Draw The Electron Configuration For A Neutral Atom Of Chlorine
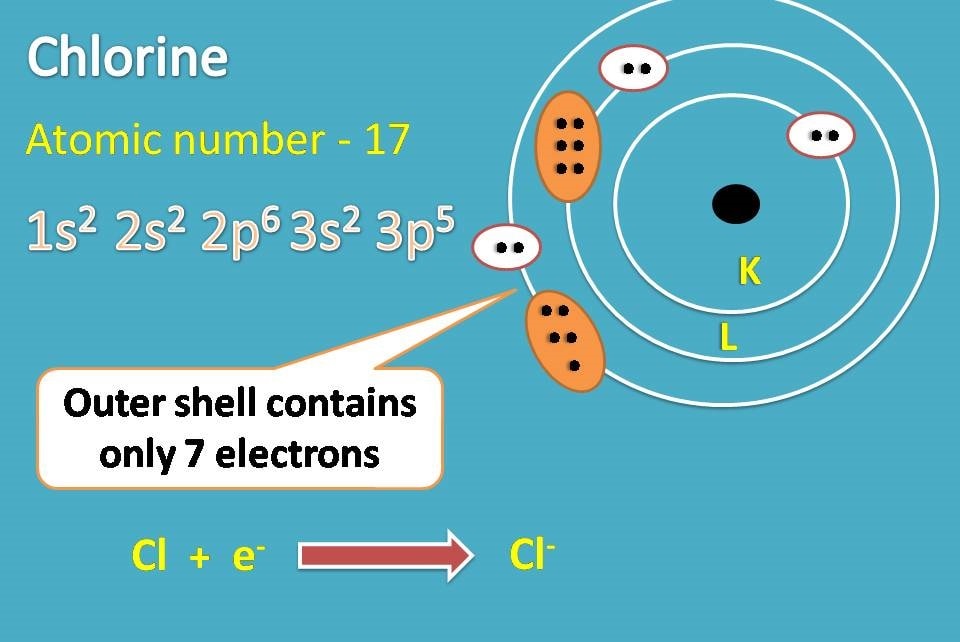
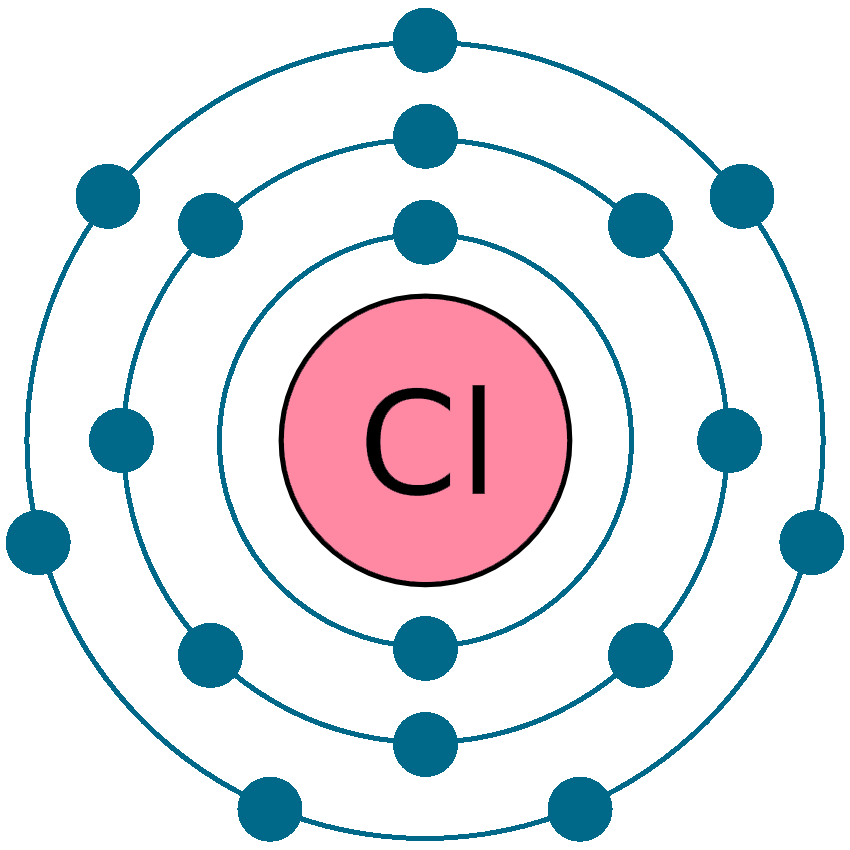
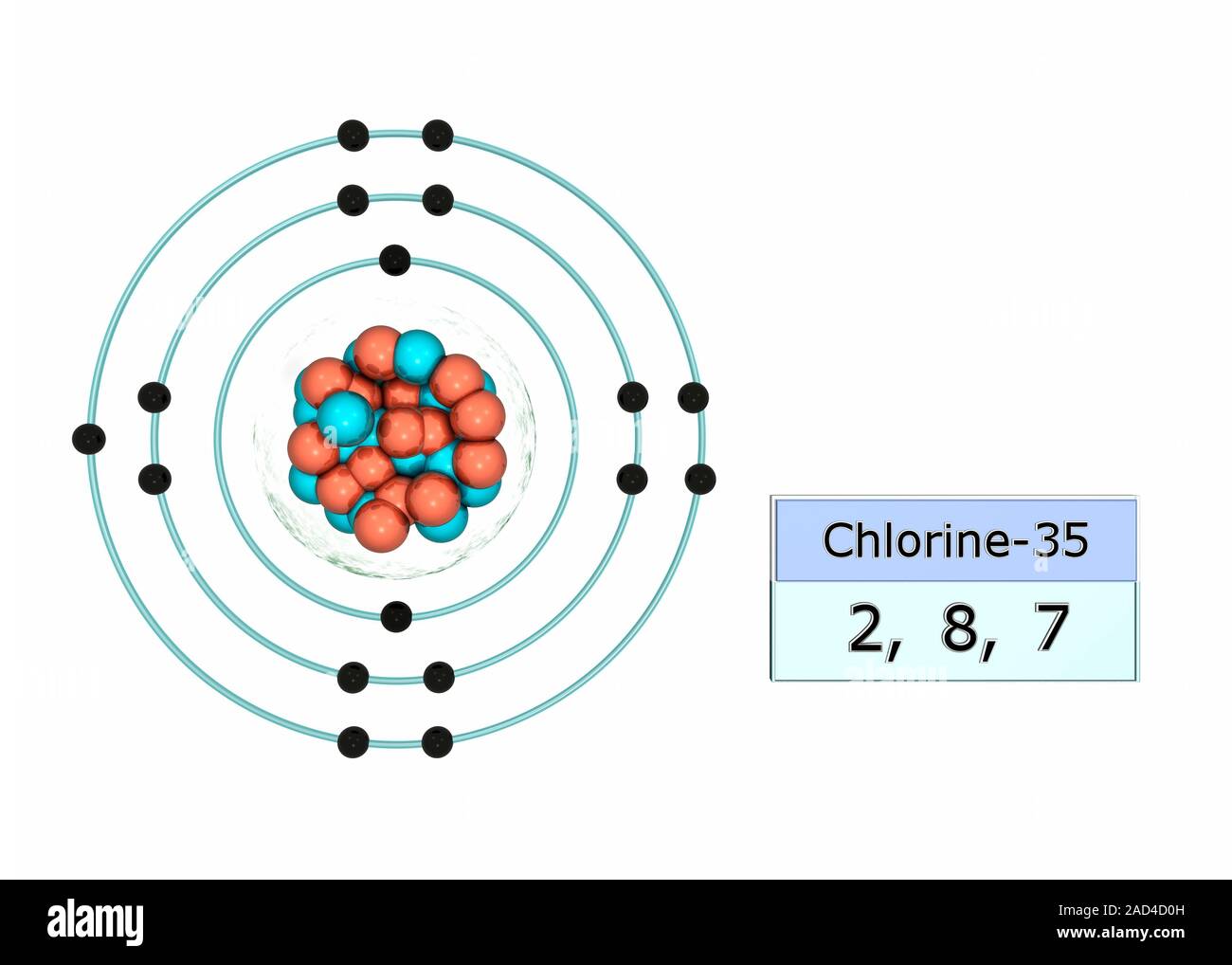
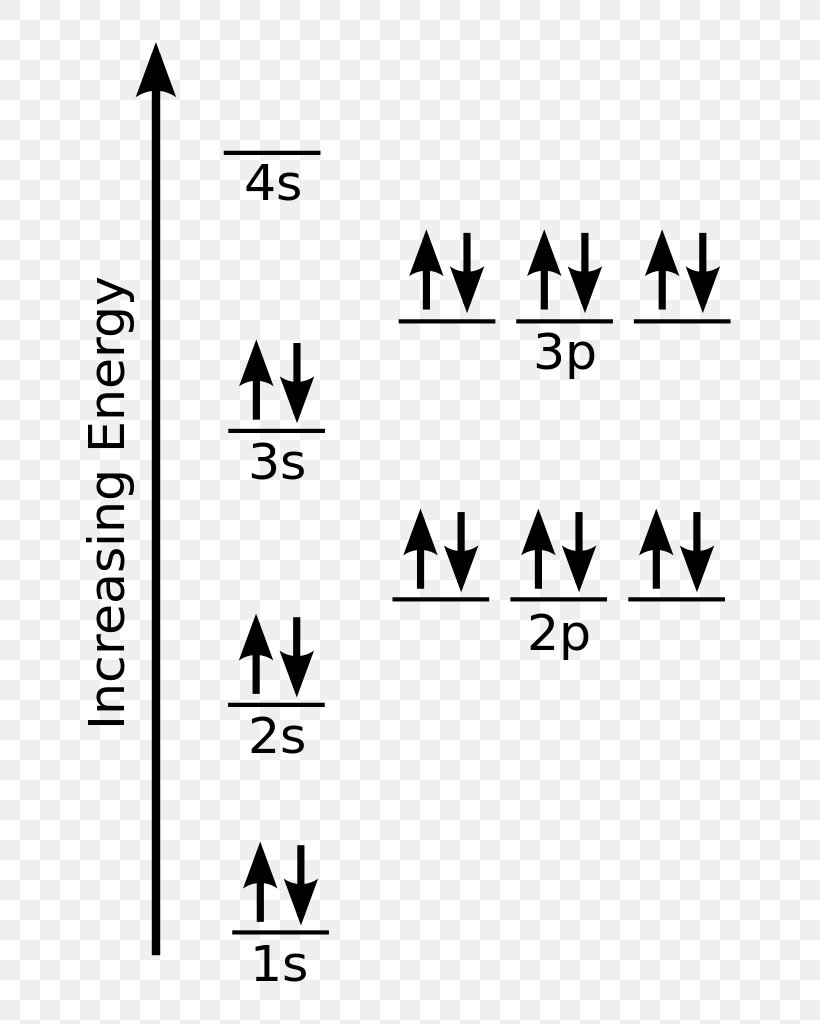
Draw The Electron Configuration For A Neutral Atom Of Chlorine - Web the arrangement of electrons in chlorine in specific rules in different orbits and orbitals is called the electron configuration of chlorine. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. The electron configuration of an atom provides information about how the electrons in it are. We add electrons to fill the outermost orbital that is. Web the electron configuration for a neutral atom of chlorine is 1s² 2s² 2p⁶ 3s² 3p⁵. The first two electrons are in the first energy level, which is closest to the nucleus. The atomic number of cl is 17. Electronic configuration of chlorine atoms. To write an electron configuration for a cation, start by writing the electron configuration for the. Web chemistry archive > atoms, compounds, and ions > introduction to the periodic table. An electron configuration diagram is a. When we write the configuration we'll. Electronic configuration of chlorine atoms. Web add an electron to the anion electron configuration. The electron configuration of an atom provides information about how the electrons in it are. So, the neutral atom of chlorine contains 17 electrons. Using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral. Since the atomic number is always equal to the number of. Web cations are formed when a neutral atom loses electrons. Chlorine has an atomic number of 17, which. Web add an electron to the anion electron configuration. Counting valence electrons for main group elements. Since the arrangement of the periodic table is based on the electron. Electronic configuration of chlorine atoms. What is the electron configuration of a neutral chlorine atom? Using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral. Web the atomic number of chlorine, cl = 17. We add electrons to fill the outermost orbital that is. The element atomic number and name are listed. Web in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Web the electron configuration for a neutral atom of chlorine is 1s² 2s² 2p⁶ 3s² 3p⁵. The element atomic number and name are listed. Web add an electron to the anion electron configuration. So, the neutral atom of chlorine contains 17 electrons. Using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral. What is the electron configuration of a neutral chlorine atom? Since the atomic number is always equal to the number of. To write an electron configuration for a cation, start by writing the electron configuration for the. Web chemistry archive > atoms, compounds, and ions > introduction to the periodic table. When we write the configuration we'll. A neutral chlorine atom has 17. Web the neutral atom chlorine (z=17), for instance has 17 electrons. Web add an electron to the anion electron configuration. We add electrons to fill the outermost orbital that is. The first two electrons are in the first energy level, which is closest to the nucleus. When we write the configuration we'll. Chlorine has 17 electrons because its atomic number is 17. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The first two electrons are in the first energy level, which is. Since the atomic number is always equal to the number of. Web the arrangement of electrons in chlorine in specific rules in different orbits and orbitals is called the electron configuration of chlorine. Counting valence electrons for main group elements. Electronic configuration of chlorine atoms. We add electrons to fill the outermost orbital that is. Electronic configuration of chlorine atoms. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. Since the arrangement of the periodic table is based on the electron. When we write the configuration we'll. The electron configuration of chlorine is 3s. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. Counting valence electrons for main group elements. Web add an electron to the anion electron configuration. Based on the periodic table, the atomic number ( z of chlorine is 17. Web the arrangement of electrons in chlorine in specific rules in different orbits and. The element atomic number and name are listed. The electron configuration of an atom provides information about how the electrons in it are. Web thus, the electron configuration of neutral chlorine atoms is 1s 2 2s 2 2p 6 3s 2 3p 5. Based on the periodic table, the atomic number ( z of chlorine is 17. Using figure \(\pageindex{2}\). The element atomic number and name are listed. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Chlorine has 17 electrons because its atomic number is 17. Web in order to write the chlorine electron configuration we first. Chlorine has 17 electrons because its atomic number is 17. Web the atomic number of chlorine, cl = 17. Web add an electron to the anion electron configuration. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Web the atomic number of chlorine, cl = 17. Web in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Full ground state electron configuration: Web the electron configuration of a chlorine atom (#cl#) is as follows: Electronic configuration of chlorine atoms. Based on the periodic table, the atomic number ( z of chlorine is 17. Chlorine has two isotopes, cl35. Web the arrangement of electrons in chlorine in specific rules in different orbits and orbitals is called the electron configuration of chlorine. The electron configuration of chlorine is 3s. To write an electron configuration for a cation, start by writing the electron configuration for the. Since the arrangement of the periodic table is based on the electron. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. Electronic configuration of chlorine atoms. For cl −, it will be. What is the electron configuration of a neutral chlorine atom? Chlorine has 17 electrons because its atomic number is 17.Chlorine Periodic Table Electron Configuration Elcho Table
[ANSWERED] The electron dot diagram for a neutral atom of chlorine Kunduz
Solved Draw the electron configuration for a neutral atom of
Draw the atomic structure of a chlorine ion Brainly.in
Draw The Electron Configuration For A Neutral Atom Of Chlorine
Chlorine Facts
draw atomic structure of chlorine Brainly.in
Chlorine Electron Dot Diagram
Chlorine Cl (Element 17) of Periodic Table NewtonDesk
Chlorine electron configuration. Illustration of the atomic structure
The Element Atomic Number And Name Are Listed.
So, The Neutral Atom Of Chlorine Contains 17 Electrons.
Web The Neutral Atom Chlorine (Z=17), For Instance Has 17 Electrons.
When We Write The Configuration We'll.
Related Post:
![[ANSWERED] The electron dot diagram for a neutral atom of chlorine Kunduz](https://media.kunduz.com/media/sug-question/raw/56588720-1659291128.051106.jpeg?h=512)