Draw The Electron Configuration For A Neutral Atom Of Manganese
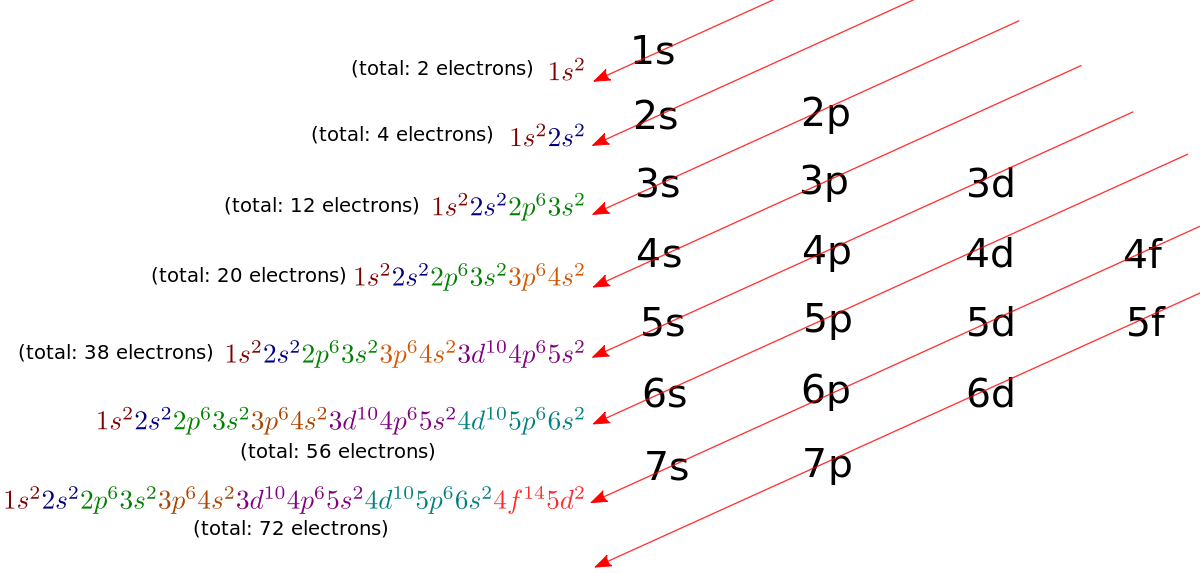
Draw The Electron Configuration For A Neutral Atom Of Manganese - Identify and explain exceptions to predicted electron configurations for atoms and ions. Web the electron configuration of manganese ion (mn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3. Write the electron configuration for the neutral atom and then determine the number of electrons that are lost to form the cation. Write the electron configuration using the aufbau principle, which states that electrons fill the lowest energy levels first. Web a quick glance at the periodic table tells me z=25.and we merely follow the aufbau principle. mn, z=25, 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (5). Draw the electron configuration for a neutral atom of manganese. Now the electrons in an atom in the ground state are arranged in different shells and subshells the following sequence according to the diagonal rule. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. The oxidation state of the element changes depending on the bond formation. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Hence the number of electrons in neutral manganese atoms is 25. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Now the electrons in an atom in the ground state are arranged in different shells and subshells the following sequence according to the diagonal rule. Draw the electron configuration for a neutral atom of manganese. Web a quick glance at the periodic table tells me z=25.and we merely follow the aufbau principle. mn, z=25, 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (5). Web today in this video, we will help you determine the electron configuration for the manganese element. It denotes a full s orbital. Manganese (mn) has an atomic number of 25, which means it has 25 electrons in its neutral state. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Web a quick glance at the periodic table tells me z=25.and we merely follow the aufbau principle. mn, z=25, 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (5). We first need to find the number of electrons for the mn. Web to write the configuration for the manganese ions, first we need to write the electron configuration for just manganese (mn). Now the electrons in an atom in the ground state are arranged in different shells and subshells the following sequence according to the diagonal rule. Your solution’s ready to go! Draw the electron configuration for a neutral atom of manganese energy. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Draw the electron configuration for a neutral atom of manganese. Web today in this video, we will help you determine the electron configuration for the manganese element. What is the name of this atom? Write the electron configuration using the aufbau principle, which states that electrons fill the lowest energy levels first. Your solution’s ready to go! Noble gases have filled energy levels and stable electron configurations. Now the electrons in an atom in the ground state are arranged in different shells and subshells the following sequence according to the diagonal rule. What is. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Web the electron configuration of a neutral atom of manganese (mn) is obtained by placing the electrons in the lowest energy levels first, moving up to higher energy levels as. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. Mn has an atomic number of 25. Web a quick glance at the periodic table tells me z=25.and we merely follow the aufbau principle. mn, z=25, 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (5). Now the electrons in an atom in the ground state are arranged in different shells and subshells the following sequence. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Your solution’s ready to go! Draw the electron configuration for a neutral atom of manganese. Web a quick glance at the periodic table tells me z=25.and we merely. Draw the electron configuration for a neutral atom of manganese. Justify the observed charge of ions to their electronic configuration. And we could abbreviate this to. Web a quick glance at the periodic table tells me z=25.and we merely follow the aufbau principle. mn, z=25, 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (5). Web today in this video, we will. We describe an electron configuration with a symbol that contains three pieces of information ( figure 6.25 ): And we could abbreviate this to. We first need to find the number of electrons for the mn. Write the electron configuration using the aufbau principle, which states that electrons fill the lowest energy levels first. Web an electrically neutral atom has. Now the electrons in an atom in the ground state are arranged in different shells and subshells the following sequence according to the diagonal rule. Predict the charge of common metallic and nonmetallic elements, and write their electron configurations. Draw the electron configuration for a neutral atom of manganese. Justify the observed charge of ions to their electronic configuration. Your. Web today in this video, we will help you determine the electron configuration for the manganese element. Identify and explain exceptions to predicted electron configurations for atoms and ions. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Web. Web a quick glance at the periodic table tells me z=25.and we merely follow the aufbau principle. mn, z=25, 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^ (5). Manganese (mn) has an atomic number of 25, which means it has 25 electrons in its neutral state. Write the electron configuration using the aufbau principle, which states that electrons fill the lowest. Justify the observed charge of ions to their electronic configuration. And we could abbreviate this to. This is the electron configuration of helium; Write the electron configuration for the neutral atom and then determine the number of electrons that are lost to form the cation. Web the electron configuration of an element is the arrangement of its electrons in its. What is the name of this atom? Manganese (mn) has an atomic number of 25, which means it has 25 electrons in its neutral state. Web the electron configuration of a neutral atom of manganese (mn) is obtained by placing the electrons in the lowest energy levels first, moving up to higher energy levels as necessary. Web determine the electron configuration of ions. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry. Draw the electron configuration for a neutral atom of manganese. Web the electron configuration of manganese, atomic number 25, is #1s^22^22p^63s^23p^63d^54s^2#. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle. We describe an electron configuration with a symbol that contains three pieces of information ( figure 6.25 ): Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Web learn about the manganese electron configuration here and make the systematic learning of the chemical element. Mn has an atomic number of 25. Web in a neutral atom, the number of electrons is equal to the number of protons in the atom i.e., the atomic number of the element. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. Draw the electron configuration for a neutral atom of manganese energy.Manganese Electron Configuration How to Write the Electron
Symbol and electron diagram for Manganese illustration Stock Vector
Manganese Atom Science Notes and Projects
Draw The Electron Configuration For A Neutral Atom Of Manganese
Atoms Diagrams Electron Configurations of Elements
SOLVED Draw the electron configuration for a neutral atom of manganese
Manganese Electron Configuration Manganese Orbital Diagram Insight
What is the electron configuration for a neutral atom of manganese?
Draw the electron configuration for a neutral atom of mangan Quizlet
Draw The Electron Configuration For A Neutral Atom Of Manganese
Determine The Atomic Number Of Manganese From The Periodic Table:
Identify And Explain Exceptions To Predicted Electron Configurations For Atoms And Ions.
Web Today In This Video, We Will Help You Determine The Electron Configuration For The Manganese Element.
Your Solution’s Ready To Go!
Related Post:

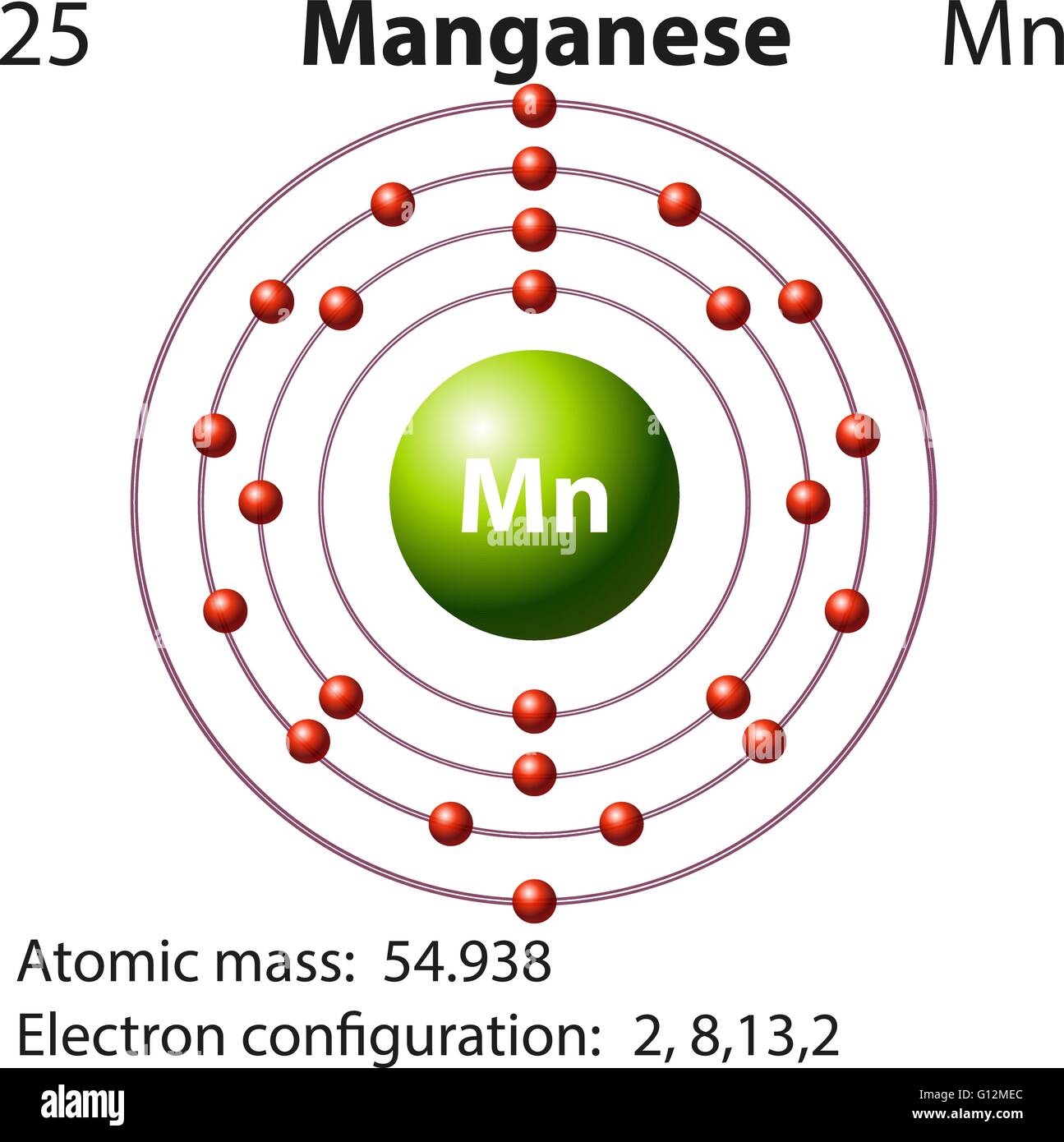


/Manganese-58b602295f9b5860464c4532.jpg)