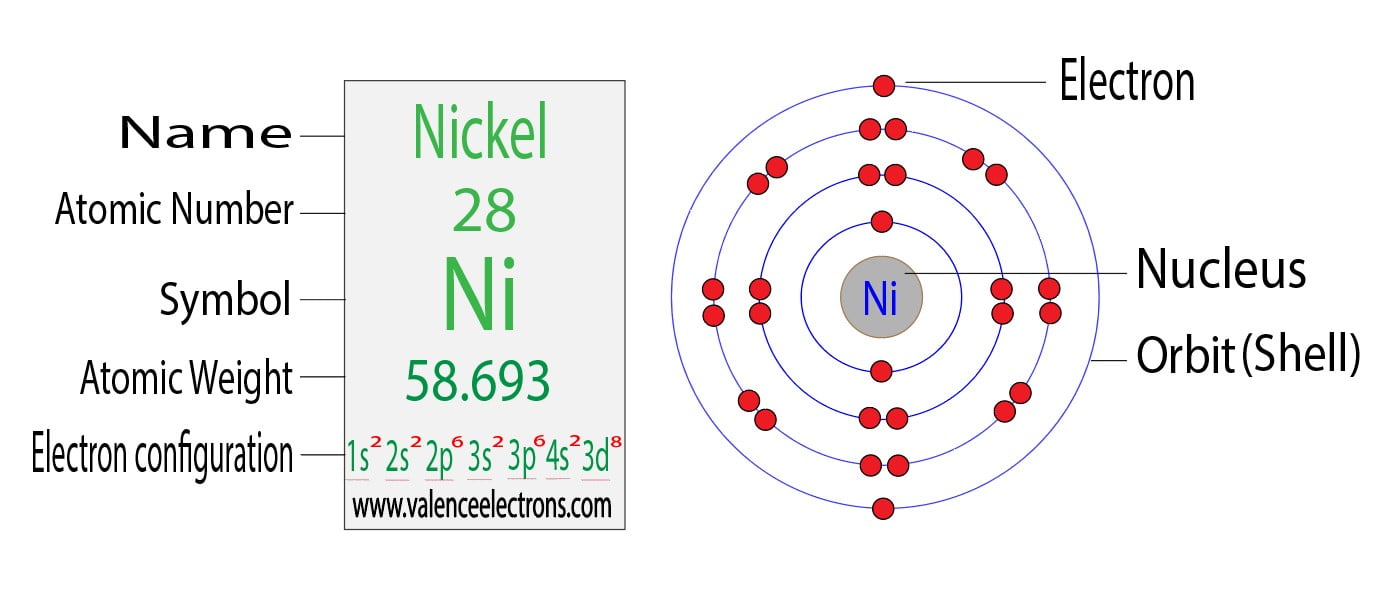
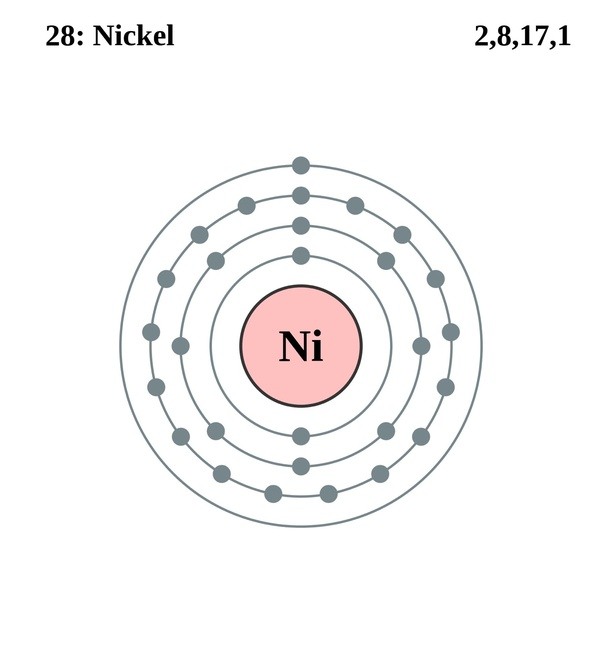
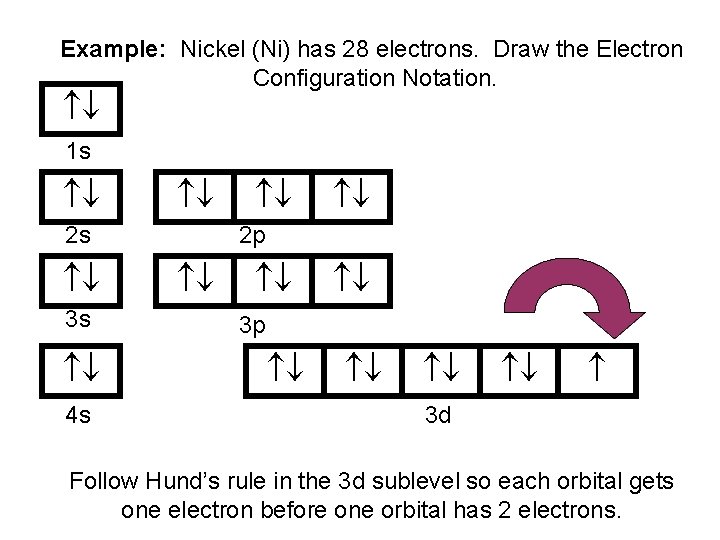
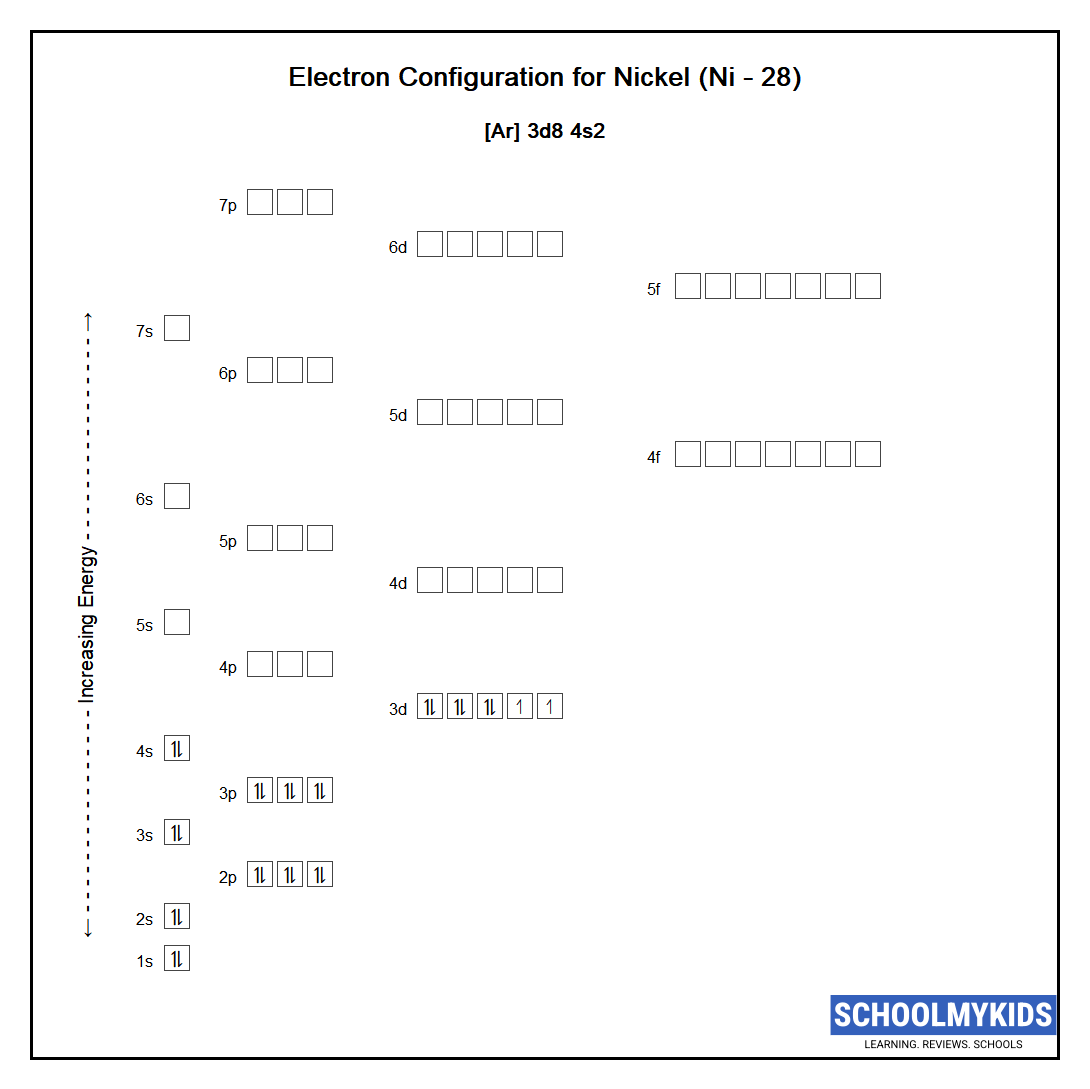
Draw The Electron Configuration For A Neutral Atom Of Nickel
Draw The Electron Configuration For A Neutral Atom Of Nickel - Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: To find the electron configuration for a. Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in. The shorthand electron configuration (or noble gas configuration) as well as. The neutral nickel atom therefore must have 2 8 electrons to accommodate according to the usual. Nickel is in the 4th energy level, d block, 7th column, this means that the electron configuration will end 3d8 with the d orbital being one level lower than. Your solution’s ready to go! Atomic number of nickel (ni) = 28. The ground state electron configuration of nickel is [ar].3d8.4s2. Draw the electron configuration for a neutral atom of nickel. The neutral nickel atom therefore must have 2 8 electrons to accommodate according to the usual. Web the ground state electron configuration of a neutral nickel atom is #[ar]3d^84s^2#. The shorthand electron configuration (or noble gas configuration) as well as. Web the correct electron configuration for a neutral atom of nickel is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁸, which corresponds to option b. This data comes from the nist atomic spectra database. Your solution’s ready to go! Nickel has a total of 28 electrons and follows the shell structure of 2.8.16.2. Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in. Web a nickel nucleus has 2 8 positively charged nuclear particles, 2 8 protons. Nickel is in the 4th energy level, d block, 7th column, this means that the electron configuration will end 3d8 with the d orbital being one level lower than. Web the electron configuration and orbital diagram for carbon are: The shorthand electron configuration (or noble gas configuration) as well as. Nickel has a total of 28 electrons and follows the shell structure of 2.8.16.2. Identify and explain exceptions to predicted electron configurations for atoms and ions. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Draw the electron configuration for a neutral atom of nickel. Where [ar] is argon with atomic. This data comes from the nist atomic spectra database. Web electron configurations describe where electrons are located around the nucleus of an atom. N i, z = 28: Atomic number of nickel (ni) = 28. Mastering the electron configuration of nickel ( [ar] 3d^8 4s^2) reveals vital insights into its atomic structure. Web when we write the configuration, we'll put all 28 electrons in orbitals around the nucleus of the nickel atom. Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Mastering the electron configuration of nickel ( [ar] 3d^8 4s^2) reveals vital insights into its atomic structure. Otherwise, write the order of the. In this video we'll use the periodic. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Web electron configurations describe where electrons are located around the nucleus of an atom. By knowing the electron configuration of an element, we can predict and. The neutral nickel atom therefore must have 2 8 electrons to. Web the neutral nickel atom therefore must have 28 electrons to accommodate according to the usual scheme: Electron configuration chart of all elements is mentioned in the table below. The neutral nickel atom therefore must have 2 8 electrons to accommodate according to the usual. By knowing the electron configuration of an element, we can predict and. Web find the. Web electron configurations describe where electrons are located around the nucleus of an atom. The neutral nickel atom therefore must have 2 8 electrons to accommodate according to the usual. N i, z = 28: Your solution’s ready to go! Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Nickel has a total of 28 electrons and follows the shell structure of 2.8.16.2. Web when we write the configuration, we'll put all 28 electrons in orbitals around the nucleus. Web the electron configuration and orbital diagram for carbon are: In this video we'll use the periodic table to help us write. This is sometimes called the bohr, or the ‘solar system’, model. Following the electron configuration rules, such. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Identify and explain exceptions to predicted electron configurations for atoms and ions. Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in. Web electron configurations describe. Web the ground state electron configuration of a neutral nickel atom is #[ar]3d^84s^2#. Web a nickel nucleus has 2 8 positively charged nuclear particles, 2 8 protons. Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. The ground state electron configuration of nickel is [ar].3d8.4s2. Web electron configurations describe. Mastering the electron configuration of nickel ( [ar] 3d^8 4s^2) reveals vital insights into its atomic structure. Web the ground state electron configuration of a neutral nickel atom is #[ar]3d^84s^2#. The neutral nickel atom therefore must have 2 8 electrons to accommodate according to the usual. Otherwise, write the order of the. Where [ar] is argon with atomic. Identify and explain exceptions to predicted electron configurations for atoms and ions. Web electron configurations describe where electrons are located around the nucleus of an atom. The ground state electron configuration of nickel is [ar].3d8.4s2. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Nickel has a total of 28 electrons and follows the shell structure of 2.8.16.2. Web when we write the configuration, we'll put all 28 electrons in orbitals around the nucleus of the nickel atom. Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. Web march 23, 2023 jay. Following the electron configuration rules, such. Your solution’s ready to go! Electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d8. To find the electron configuration for a. In this video we'll use the periodic table to help us write. Electron configuration chart of all elements is mentioned in the table below. Web the electron configuration and orbital diagram for carbon are: N i, z = 28:OneClass draw the electron configuration for a neutral atom of nickel.
Nickel Electron Configuration(Explained for Beginners)
19+ Orbital Diagram Of Nickel BrodyNaevia
Nickel Electron Configuration (Ni) with Orbital Diagram
Electron Orbital Configuration Chart
Symbol and electron diagram for nickel Royalty Free Vector
Nickel Atom Science Notes and Projects
Electron Configuration Chapter 5 Electrons have 3 levels
Nickel (Ni) Element Information, Facts, Properties, Uses Periodic
Electron Configuration For Nickel cloudshareinfo
Web The Neutral Nickel Atom Therefore Must Have 28 Electrons To Accommodate According To The Usual Scheme:
Atomic Number Of Nickel (Ni) = 28.
Web A Nickel Nucleus Has 2 8 Positively Charged Nuclear Particles, 2 8 Protons.
This Is Sometimes Called The Bohr, Or The ‘Solar System’, Model.
Related Post: