Draw The Electron Configuration For A Neutral Atom Of Sodium
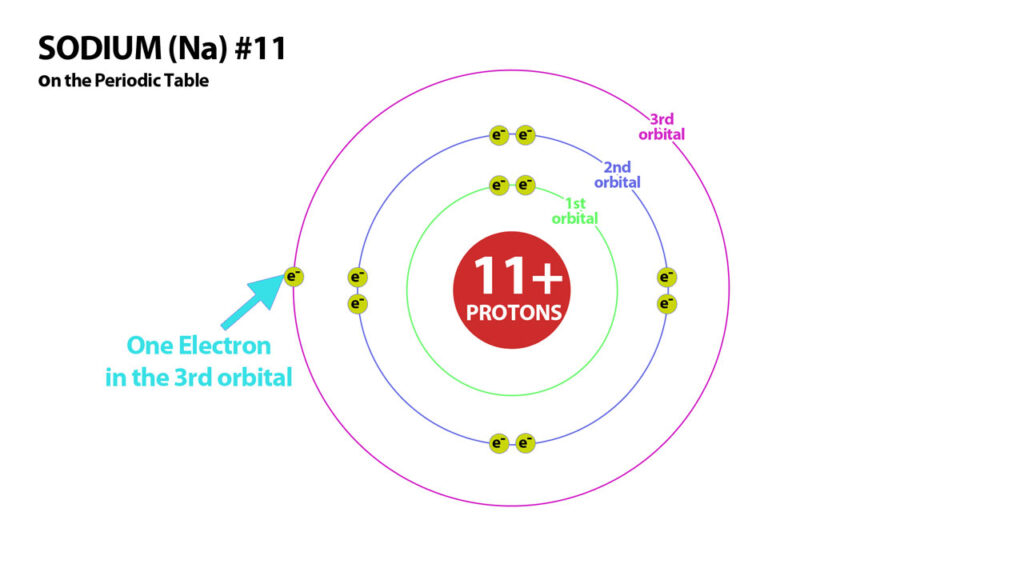
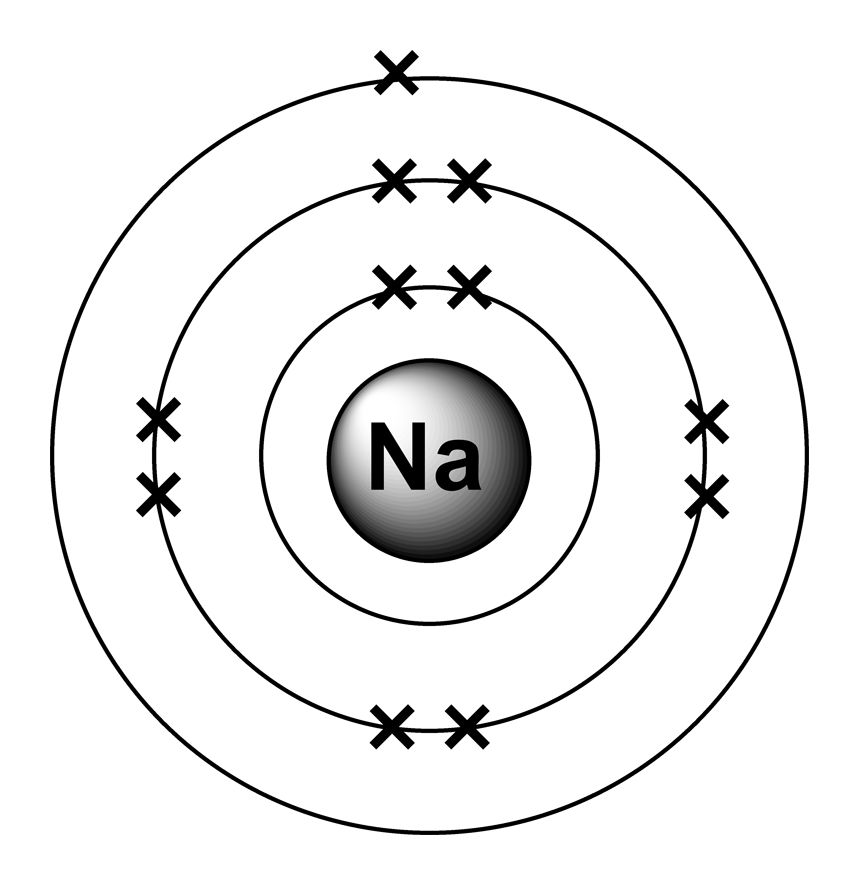
Draw The Electron Configuration For A Neutral Atom Of Sodium - Web the upper right side shows the number of electrons in a neutral atom. By knowing the electron configuration of an element, we can predict and. An electron configuration diagram is a model that depicts. Web march 23, 2023 jay. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,. Web this video shows how to draw the orbital diagram of sodium (na). Remember, a neutral atom contains the same number of protons and electrons. Web typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the. Electron configuration chart of all elements is mentioned in the table below. Web the electron configuration of a neutral sodium atom is #1s^2 2s^2 2p^6 3s^1#. It also shows how to write the electron configuration of sodium (na) and the shorthand nobl. View the full answer step 2. Web it is important in understanding how elements form chemical bonds. According to the aufbau principle, this arrangement depicts the distribution. Web typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the. Web with an atomic number of 11, a neutral sodium atom (na) has the electron configuration 1s² 2s² 2p⁶ 3s¹. An electron configuration diagram is a model that depicts. In this configuration we note that there is only one electron in the 3rd energy level. Web first, write out the electron configuration for each parent atom. Web the electron configuration of a neutral sodium atom is #1s^2 2s^2 2p^6 3s^1#. Web the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. It also shows how to write the electron configuration of sodium (na) and the shorthand nobl. Web with an atomic number of 11, a neutral sodium atom (na) has the electron configuration 1s² 2s² 2p⁶ 3s¹. Atomic number of na = 11. Remember, a neutral atom contains the same number of protons and electrons. Web this video shows how to draw the orbital diagram of sodium (na). Web draw the electron configuration for a neutral atom of sodium. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,. In this configuration we note that there is only one electron in the 3rd energy level. There are 2 steps to solve this one. Web the upper right side shows the number of electrons in a neutral atom. Web therefore, the electron configuration of sodium is 1s2 2s2 2p6 3s1. In this configuration we note that there is only one electron in the 3rd energy level. Atomic number of na = 11. For sodium (na), which has 11 electrons, the electron configuration can be. Web draw the electron configuration for a neutral atom of sodium. It also shows how to write the electron configuration of sodium (na) and the shorthand nobl. Atomic number of na = 11. Electron configuration chart of all elements is mentioned in the table below. Web typically, you need at least 8 steps to determine the electron configuration, starting with. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,. It also shows how to write the electron configuration of sodium (na) and the shorthand nobl. In this configuration we note that there is only one electron in the 3rd energy level. Web draw the electron configuration for a neutral atom of. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web this video shows how to draw the orbital diagram of sodium (na). Web the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Web it is important in understanding. Web this video shows how to draw the orbital diagram of sodium (na). Web with an atomic number of 11, a neutral sodium atom (na) has the electron configuration 1s² 2s² 2p⁶ 3s¹. According to the aufbau principle, this arrangement depicts the distribution. By knowing the electron configuration of an element, we can predict and. Web draw the electron configuration. To understand the mechanism of sodium electron configuration, you need to understand two basic things. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web therefore, the electron configuration of sodium is 1s2 2s2 2p6 3s1. Web typically, you need at least 8 steps to determine the electron configuration, starting with finding. Atomic number of na = 11. In this configuration we note that there is only one electron in the 3rd energy level. According to the aufbau principle, this arrangement depicts the distribution. It also shows how to write the electron configuration of sodium (na) and the shorthand nobl. Web march 23, 2023 jay. Web draw the electron configuration for a neutral atom of sodium. There are 2 steps to solve this one. An electron configuration diagram is a model that depicts. Web first, write out the electron configuration for each parent atom. For sodium (na), which has 11 electrons, the electron configuration can be written using the periodic table or an. There are 2 steps to solve this one. The shorthand electron configuration (or noble gas configuration) as well as. Web the electron configuration of a neutral sodium atom is #1s^2 2s^2 2p^6 3s^1#. According to the aufbau principle, this arrangement depicts the distribution. An electron configuration diagram is a model that depicts. Web first, write out the electron configuration for each parent atom. Web the electron configuration of a neutral sodium atom is #1s^2 2s^2 2p^6 3s^1#. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,. Web march 23, 2023 jay. Electron configuration chart of all elements is mentioned in the table below. Web it is important in understanding how elements form chemical bonds. According to the aufbau principle, this arrangement depicts the distribution. For sodium (na), which has 11 electrons, the electron configuration can be written using the periodic table or an. Web the upper right side shows the number of electrons in a neutral atom. This makes it easier to. Web this video shows how to draw the orbital diagram of sodium (na). View the full answer step 2. Atomic number of na = 11. The shorthand electron configuration (or noble gas configuration) as well as. Web with an atomic number of 11, a neutral sodium atom (na) has the electron configuration 1s² 2s² 2p⁶ 3s¹. Remember, a neutral atom contains the same number of protons and electrons. Web march 23, 2023 jay. Electron configuration chart of all elements is mentioned in the table below. It also shows how to write the electron configuration of sodium (na) and the shorthand nobl. An electron configuration diagram is a model that depicts. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it,.Basic Structure of Atoms Clearly Explained
Introduction to Atoms
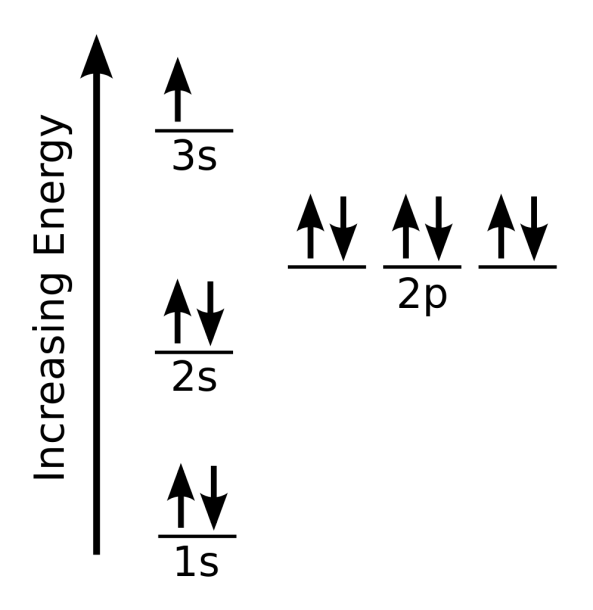
Electron configurations
Atom Diagrams Electron Configurations of the Elements
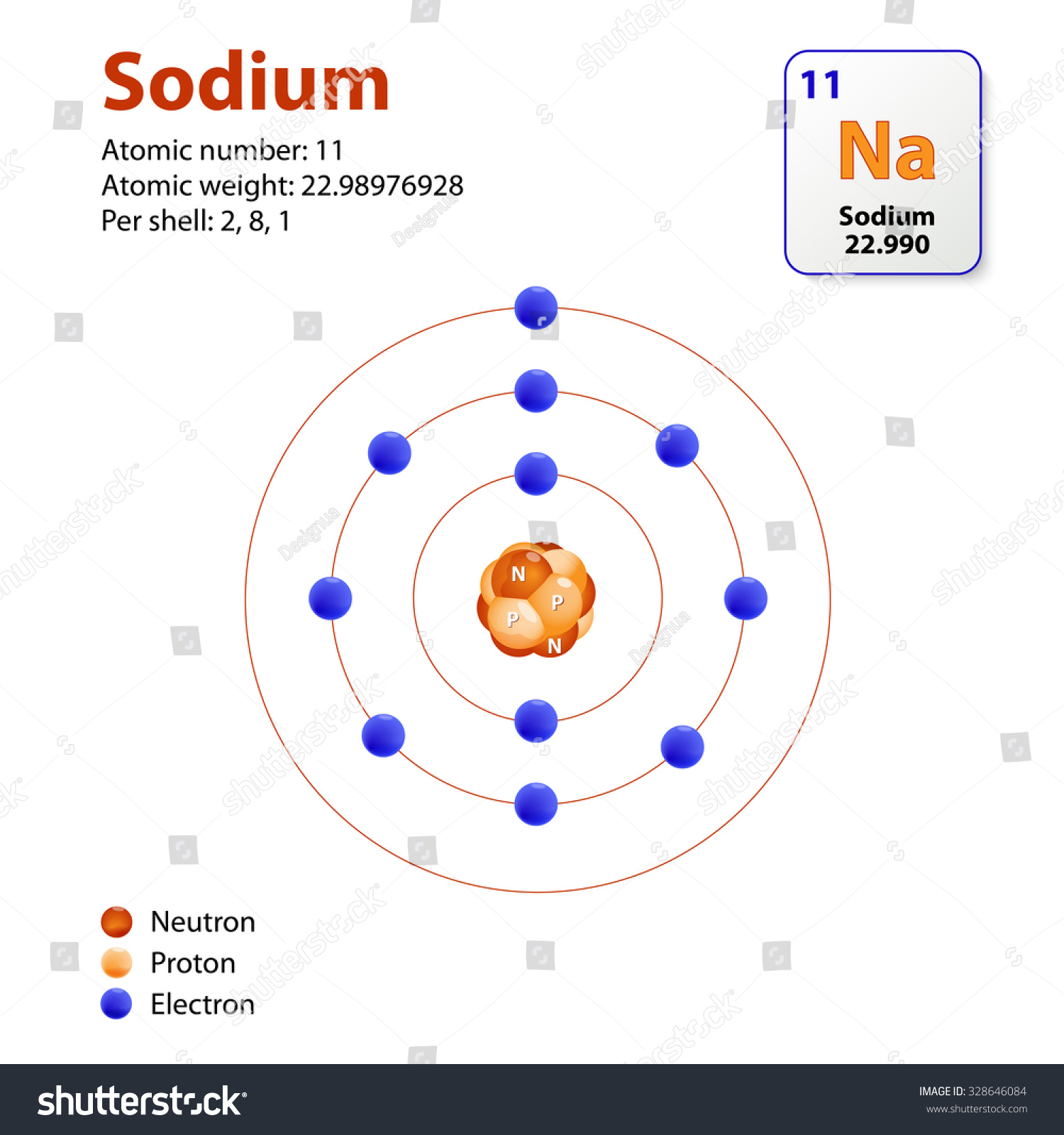
This Diagram Shows The Electron Shell Configuration For The Sodium Atom
Sodium Orbital Diagram
Sodium Electron Configuration With Full Orbital Diagram
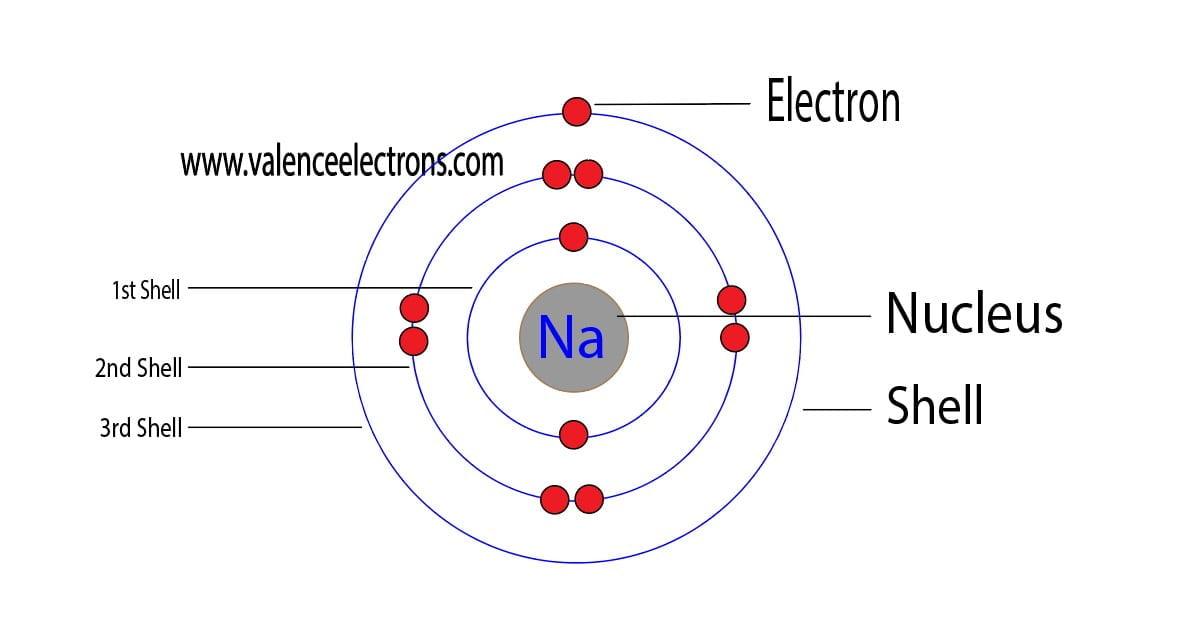
FileElectron shell 011 sodium.png Wikimedia Commons
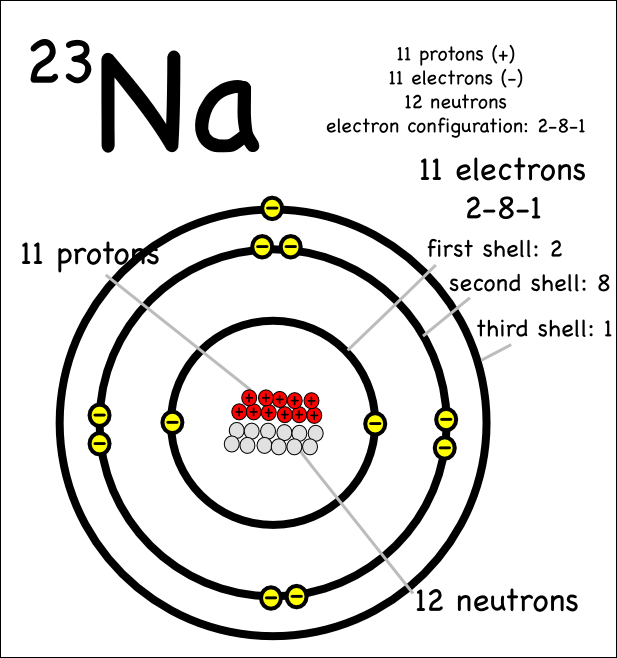
Sodium Bohr Diagram
Sodium Electron Configuration (Na) with Orbital Diagram
Web The Electron Configuration Of A Neutral Sodium Atom Is #1S^2 2S^2 2P^6 3S^1#.
Web The Configuration Notation Provides An Easy Way For Scientists To Write And Communicate How Electrons Are Arranged Around The Nucleus Of An Atom.
Web Typically, You Need At Least 8 Steps To Determine The Electron Configuration, Starting With Finding The Atomic Number By Looking At The List Of Orbitals And Understanding The.
By Knowing The Electron Configuration Of An Element, We Can Predict And.
Related Post:

:max_bytes(150000):strip_icc()/sodiumatom-58b602715f9b5860464c7a22.jpg)