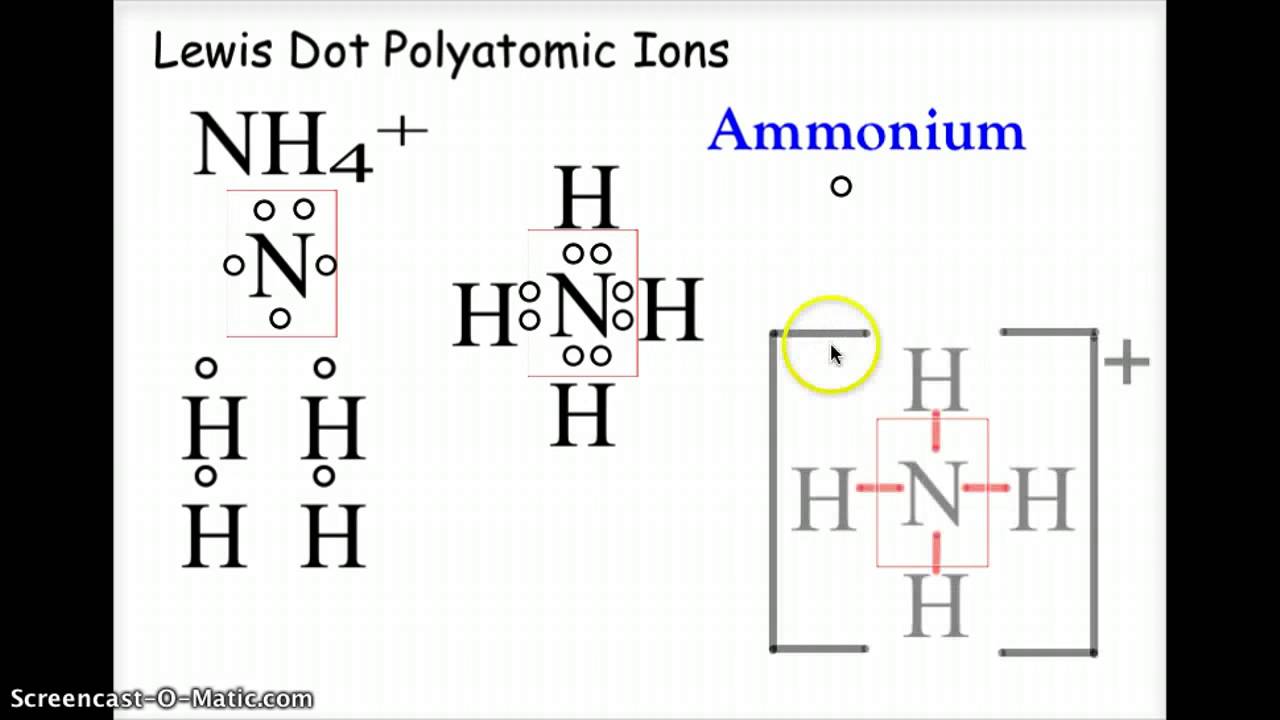
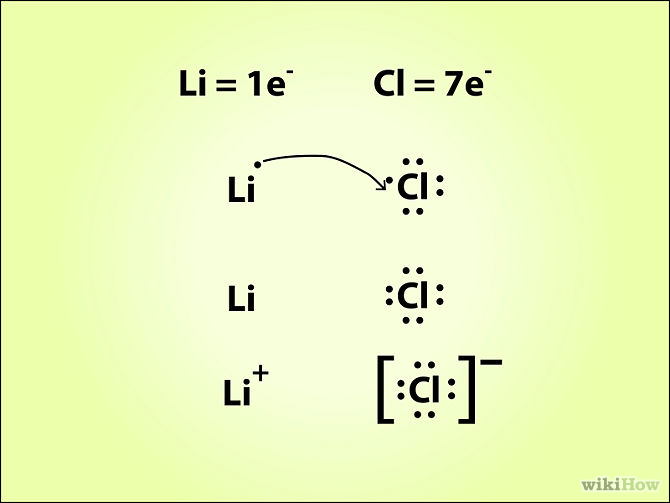
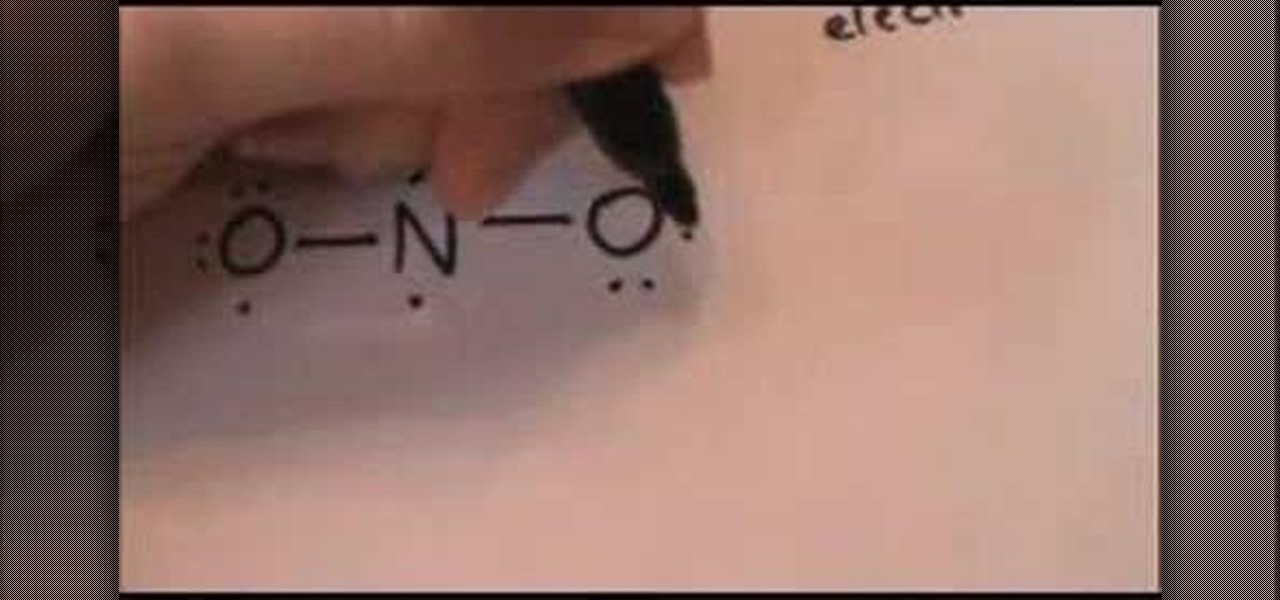
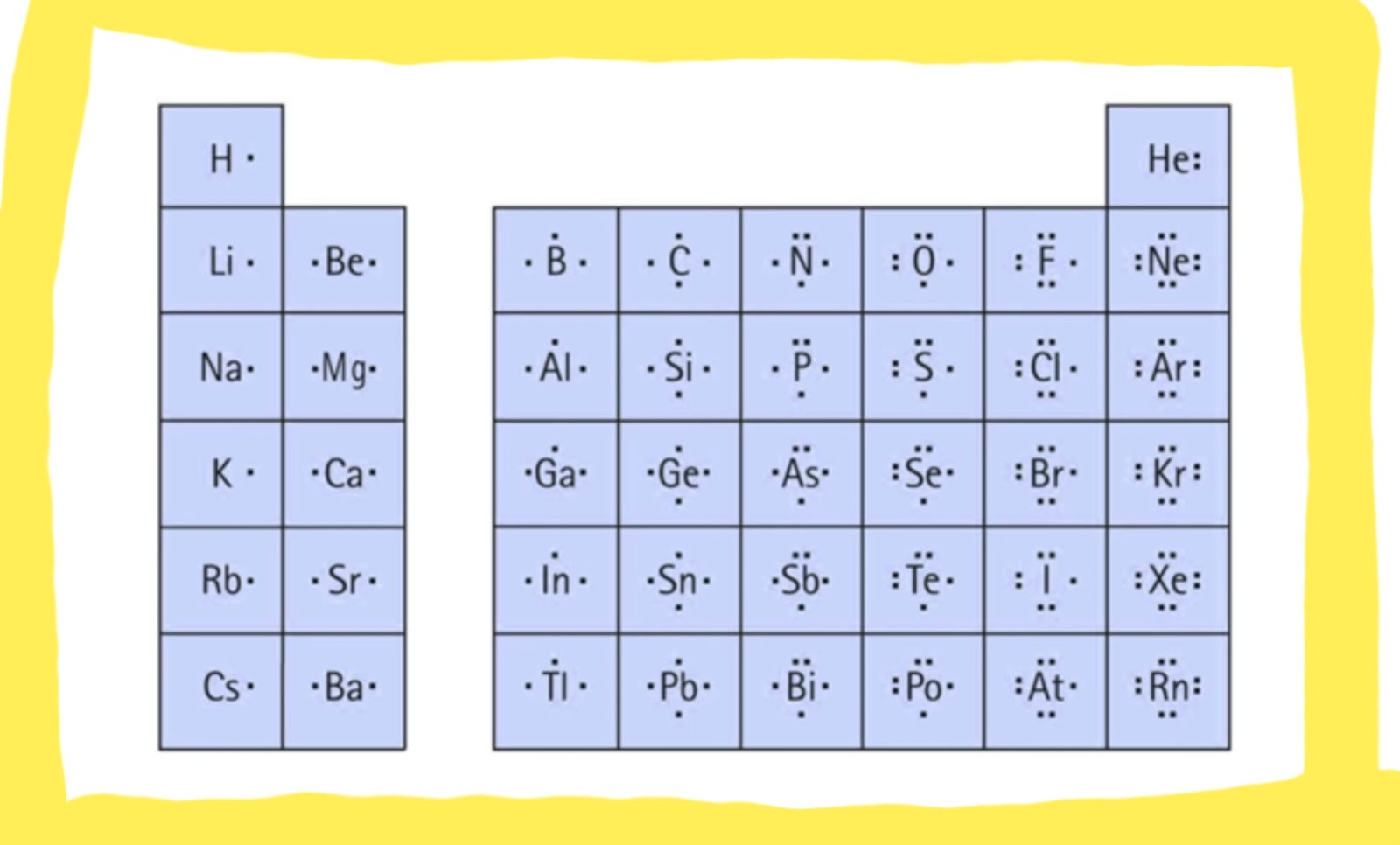
Draw The Lewis Dot Diagram For A Anion
Draw The Lewis Dot Diagram For A Anion - Understand the concept of electron gain and how it affects. How would you draw a lewis structure for an atom that has the electron. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Lone pairs, unpaired electrons, and single, double, or. Determine the number of valence electrons for hydrogen. A lewis structure is a diagram that shows the chemical bonds between atoms in a. Web we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Web first, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom. Then, determine whether the atoms are held together by a single,. Web draw the lewis dot diagram for a c− anion. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. It also represents the total number of lone pairs present. Web we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Determine the number of valence electrons for hydrogen. Since it is an anion, it means it has gained an electron. Then, determine whether the atoms are held together by a single,. The valence electron configuration for aluminum is 3 s2 3 p1. A lewis structure is a diagram that shows the chemical bonds between atoms in a. How do you draw the lewis structure of p? Here’s the best way to solve it. If the molecule is an anion, extra electrons. Web we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Since it is an anion, it means it has gained an electron. Then, determine whether the atoms are held together by a single,. Web learn how to draw the lewis dot diagram for an anion, a negatively charged ion, using simple steps and guidelines. Hydrogen has 1 valence electron. How would you draw a lewis structure for an atom that has the electron. Here’s the best way to solve it. Determine the number of valence electrons in a neutral carbon atom and then add one extra electron. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web first, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom. Web how do you draw the lewis dot structure for #cal_2#? The example is for the nitrate ion. What is the. Lewis electron dot diagrams for ions have less (for cations) or. Lewis electron dot diagrams for ions have fewer (for cations) or more (for. Then, determine whether the atoms are held together by a single,. How do you draw the lewis structure of p? Web learn how to draw the lewis dot diagram for an anion, a negatively charged ion,. Here’s the best way to solve it. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Web draw the lewis dot diagram for a c− anion. Web first, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom. What is the lewis electron dot. It also represents the total number of lone pairs present. Web learn how to draw the lewis dot diagram for an anion, a negatively charged ion, using simple steps and guidelines. The example is for the nitrate ion. Then, determine whether the atoms are held together by a single,. Understand the concept of electron gain and how it affects. How would you draw a lewis structure for an atom that has the electron. Shared pairs of electrons are drawn as lines between atoms, while lone pairs. Web lewis dot diagram is the diagram which describes the chemical bonding between atoms in a molecule. Determine the number of valence electrons for hydrogen. Lone pairs, unpaired electrons, and single, double, or. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions). Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Determine the number of valence electrons in a neutral carbon atom and then add one extra electron. Then, determine whether the atoms are held together by a single,. Lewis electron. Web how do you draw the lewis dot structure for #cal_2#? If the molecule is an anion, extra electrons. Since it is an anion, it means it has gained an electron. Web first, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom. Valence electronic structures can be visualized by. Determine the number of valence electrons in a neutral carbon atom and then add one extra electron. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Since it is an anion, it means it has gained an electron. Shared pairs of electrons are drawn as lines between atoms, while lone pairs. Hydrogen follows the. Determine the number of valence electrons in a neutral carbon atom and then add one extra electron. Understand the concept of electron gain and how it affects. A lewis symbol consists of an elemental symbol surrounded by one. The valence electron configuration for aluminum is 3 s2 3 p1. Since it is an anion, it means it has gained an. Lewis electron dot diagrams for ions have fewer (for cations) or more (for. It also represents the total number of lone pairs present. A lewis symbol consists of an elemental symbol surrounded by one. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web lewis dot diagram is the diagram which describes. What is the lewis electron dot diagram for each element? Determine the number of valence electrons in a neutral carbon atom and then add one extra electron. Web first, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Then, determine whether the atoms are held together by a single,. Web we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. How would you draw a lewis structure for an atom that has the electron. Hydrogen follows the doublet rule, so you can pair the two electrons up. It also represents the total number of lone pairs present. Web lewis dot diagram is the diagram which describes the chemical bonding between atoms in a molecule. The valence electron configuration for aluminum is 3 s2 3 p1. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions). Hydrogen has 1 valence electron. Lewis electron dot diagrams for ions have fewer (for cations) or more (for. Web here are the steps to draw a lewis structure. Understand the concept of electron gain and how it affects.How To Draw Lewis Structures For Ionic Compounds
Lewis Structure Of Ions
How to Draw the Lewis Dot Structure for CHO (Formyl anion) YouTube
Question 023ed Socratic
SOLVED Draw the Lewis dot diagram for a H anion
Lewis Dot Diagram For Ionic Compounds
BohrRutherford Diagrams & Lewis Dot Diagrams Eve Wongworakul
How to Draw a Lewis dot structure for nutrite anion « Science
How to Draw Lewis Dot Structure
Lewis Dot Structures of Atoms and Ions YouTube
Web Lewis Electron Dot Diagrams Use Dots To Represent Valence Electrons Around An Atomic Symbol.
Web Learn How To Draw The Lewis Dot Diagram For An Anion, A Negatively Charged Ion, Using Simple Steps And Guidelines.
Lone Pairs, Unpaired Electrons, And Single, Double, Or.
How Do You Draw The Lewis Structure Of P?
Related Post: