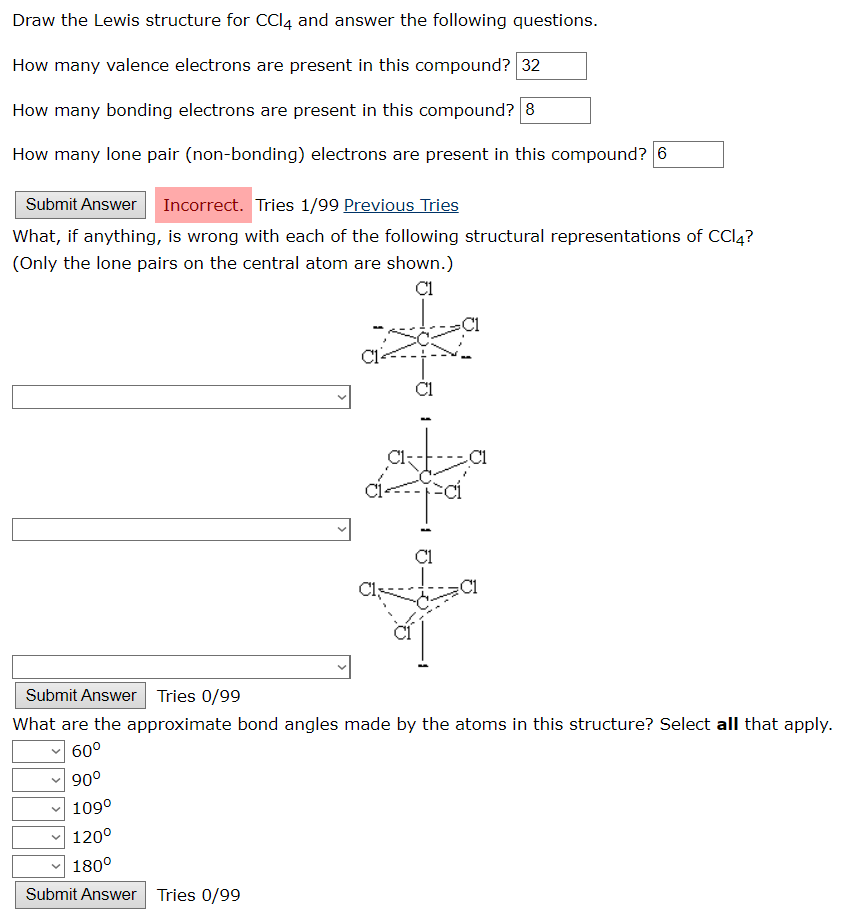
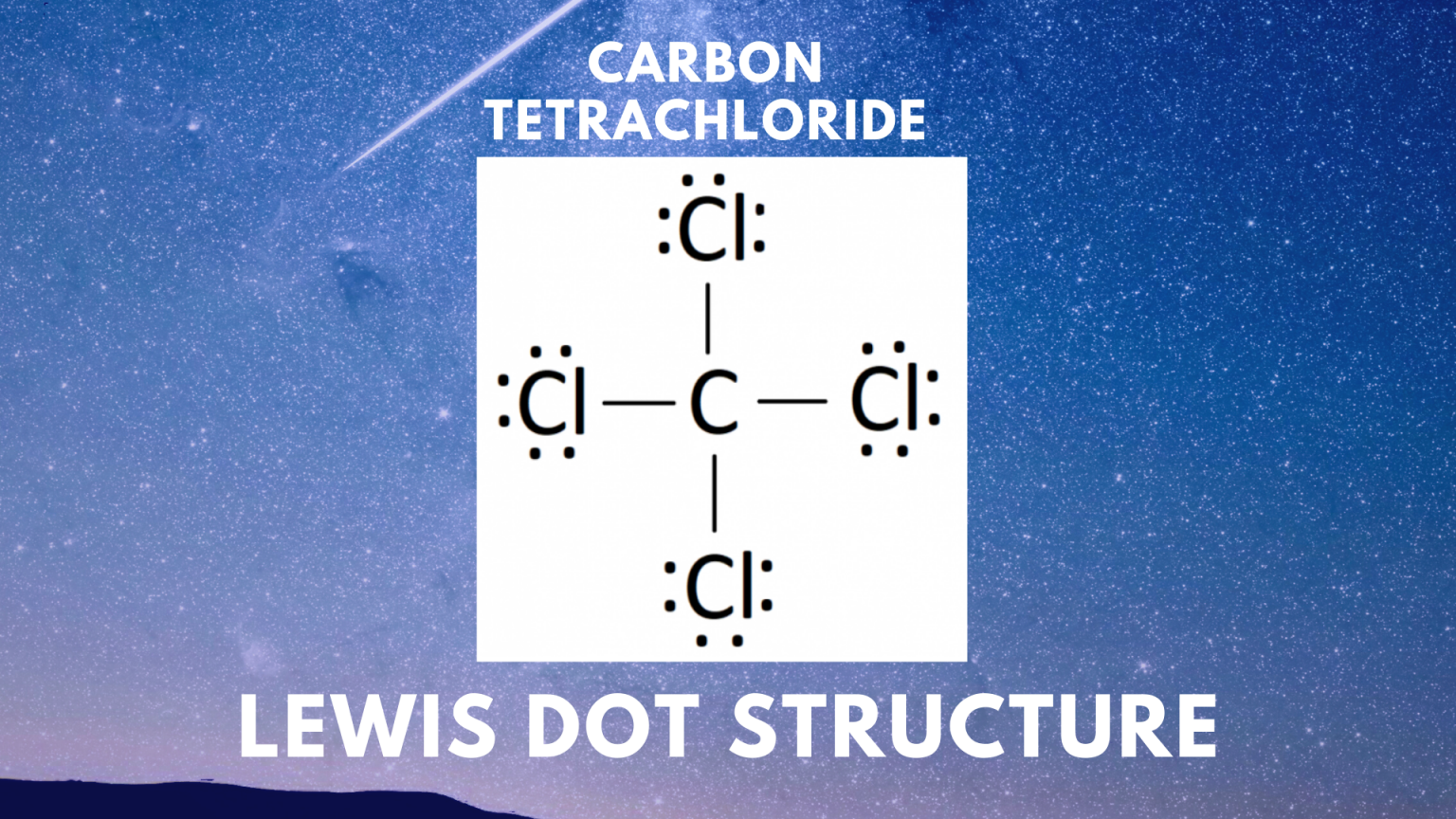
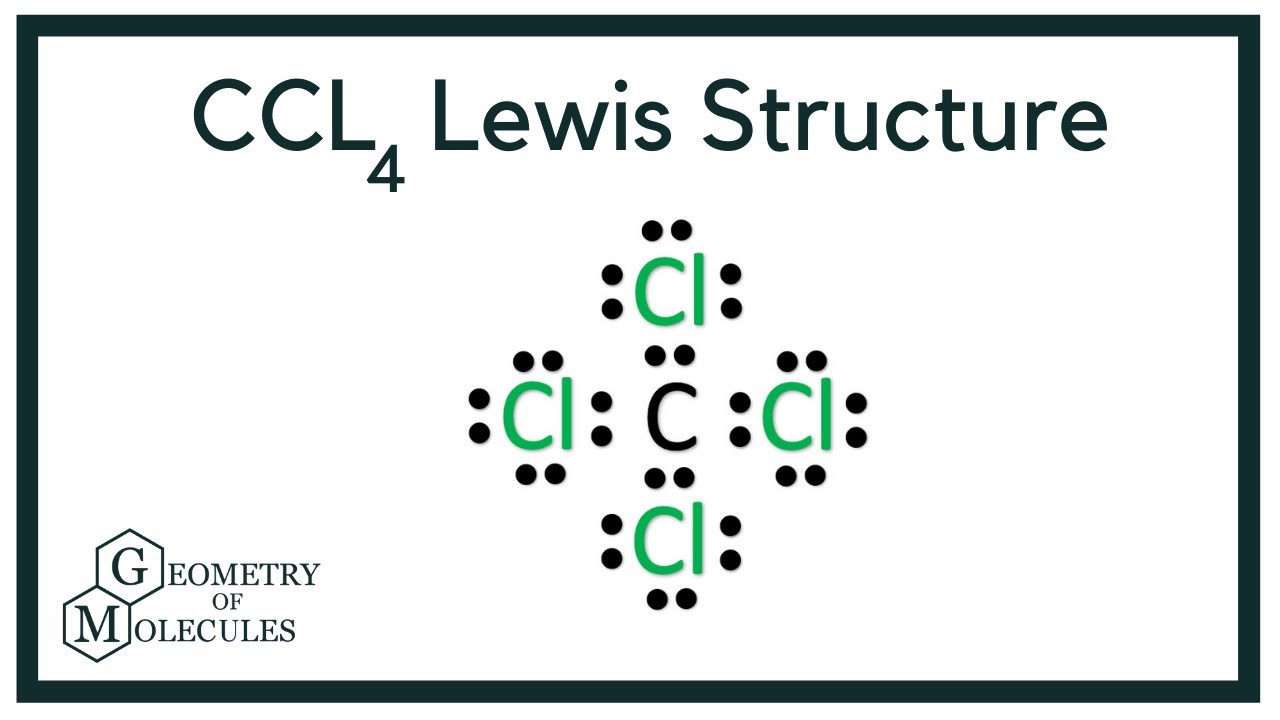
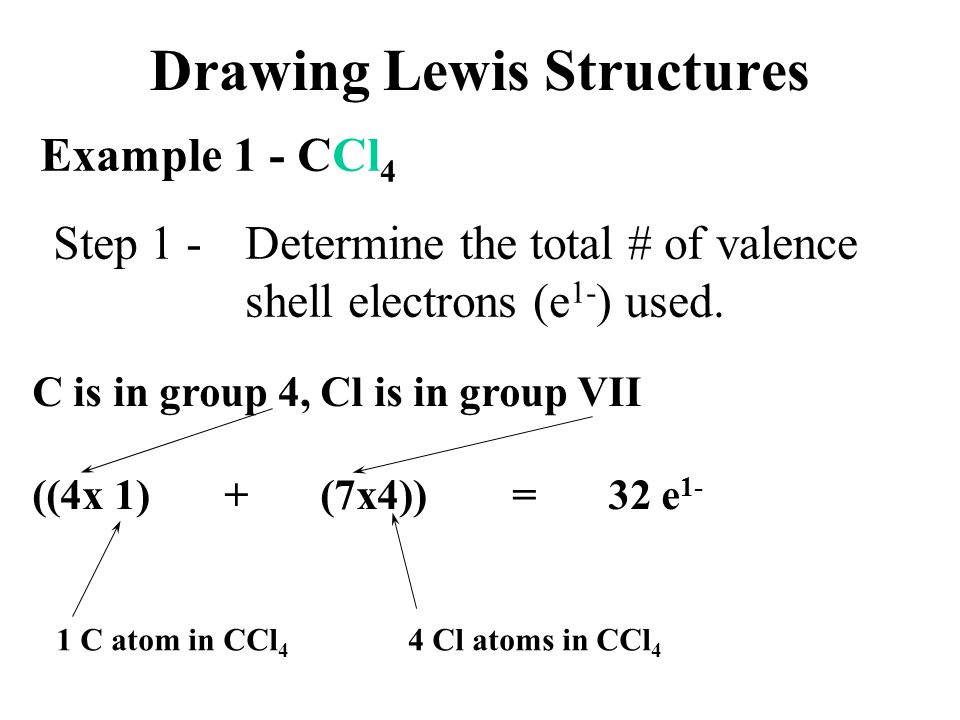
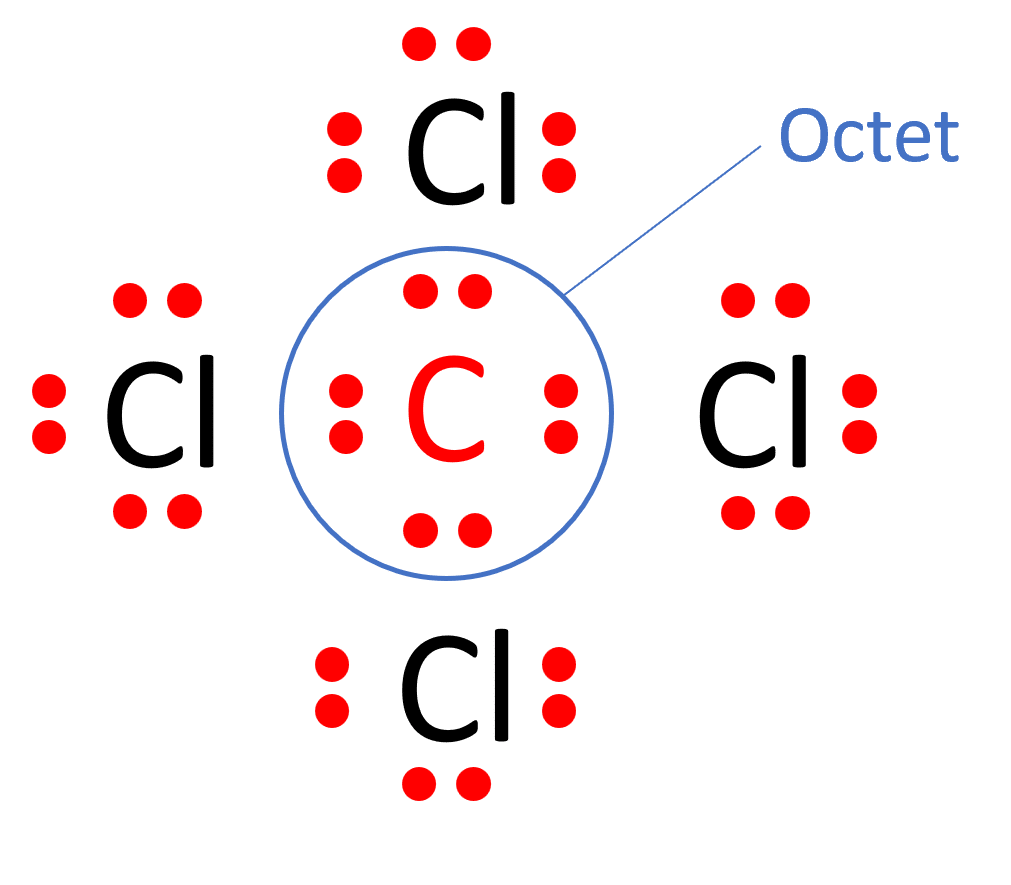
Draw The Lewis Structure For Ccl4
Draw The Lewis Structure For Ccl4 - That will be the least electronegative atom (#c#). Is the molecule polar or nonpolar? What is the molecular geometry of this compound? Begin by determining the total number of valence electrons. Web to properly draw the ccl 4 lewis structure, follow these steps: The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Chlorine has 7 valence electrons, but we have 4 chlorines so let's multiply that by 4. Determine total electrons pairs as lone pairs and bonds; Web draw lewis structures for covalent compounds. Find total number of electrons of the valance shells of carbon atom and chlorine atoms; Mark lone pairs on atoms That will be the least electronegative atom (#c#). Chlorine has 7 valence electrons, but we have 4 chlorines so let's multiply that by 4. Web draw lewis structures for covalent compounds. Draw lewis structures for covalent compounds. Web there are several steps to complete the lewis structure of ccl 4. Find center atom and draw basic skeletal structure; Here are the steps that i follow when drawing a lewis structure. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. I also go over hybridization, shape and bond angle. Web a ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. The following procedure can be used to draw lewis structure for simple molecules. Web in this tutorial, we’ll illustrate the steps to draw the lewis structure for tetrachloromethane, denoted as ccl4. Draw lewis structures for covalent compounds. Determine total electrons pairs as lone pairs and bonds; Web for the lewis structure of ccl4 first, let’s calculate the total valence electrons. Mark lone pairs on atoms Web the lewis structure for carbon tetrachloride (ccl4) involves each of the four chlorines (cl) forming a single bond with the central carbon (c). That will be the least electronegative atom (#c#). Web here, i have explained 6 simple steps to draw the lewis dot structure of ccl4 (along with images). Web a video explanation of how to draw the lewis dot structure for carbon tetrachloride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape,. Here are the steps that i follow when drawing a lewis structure. Web. The lewis structure for ccl4 is a commonly tested lewis struc. Web for the lewis structure of ccl4 first, let’s calculate the total valence electrons. Carbon is in group 4 or 14, so it has 4. Web the lewis structure for carbon tetrachloride (ccl4) involves each of the four chlorines (cl) forming a single bond with the central carbon (c).. Each step of drawing is explained in detail in this tutorial. Web a video explanation of how to draw the lewis dot structure for carbon tetrachloride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape,. Web in this tutorial, we’ll illustrate the steps to draw the lewis structure for tetrachloromethane, denoted as ccl4. Web to properly. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on. Web there are several steps to complete the lewis structure of ccl 4. Determine total electrons pairs as lone pairs and bonds; Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Draw lewis structures depicting the bonding in simple molecules As there are four molecules of chlorine, we will calculate the. The remaining electrons in each chlorine atom form lone. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! The following procedure can be used to draw lewis structure for simple molecules. That will be the least electronegative atom (#c#). I also go over hybridization, shape and bond angle. Web ccl4 lewis structure has a carbon atom (c) at the center which is surrounded by four chlorine atoms (cl). Carbon has four valence electrons and each chlorine atom has seven valence electrons. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Web i quickly take you through how to draw. What is the molecular geometry of this compound? Each step of drawing is explained in detail in this tutorial. We'll start by looking at the valence electrons. Web a video explanation of how to draw the lewis dot structure for carbon tetrachloride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape,. Web this is all about. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Find center atom and draw basic skeletal structure; Web a video explanation of how to draw the lewis dot structure for carbon tetrachloride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape,. Begin by determining the total number of. We'll start by looking at the valence electrons. Find total number of electrons of the valance shells of carbon atom and chlorine atoms; Web the lewis structure for carbon tetrachloride (ccl4) involves each of the four chlorines (cl) forming a single bond with the central carbon (c). Web ccl4 lewis structure has a carbon atom (c) at the center which. That will be the least electronegative atom (#c#). Web a video explanation of how to draw the lewis dot structure for carbon tetrachloride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape,. Chlorine has 7 valence electrons, but we have 4 chlorines so let's multiply that by 4. Draw the lewis structure for ccl4. Determine total electrons pairs as lone pairs and bonds; Web ccl4 lewis structure has a carbon atom (c) at the center which is surrounded by four chlorine atoms (cl). I also go over hybridization, shape and bond angle. There are 4 single bonds between the carbon atom (c) and each chlorine atom (cl). #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary. Lewis structure of ccl4 contains four single bonds between the carbon (c) atom and each chlorine (cl) atom. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. Web let's do the lewis structure for ccl4, carbon tetrachloride, sometimes just called carbon tet. Web in this tutorial, we’ll illustrate the steps to draw the lewis structure for tetrachloromethane, denoted as ccl4. Your solution’s ready to go! Web drawing lewis structures for molecules with one central atom: Web a ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded compounds.Solved Draw the Lewis structure for CCl4 and answer the
CCl4 Lewis Structure Science Trends
CCl4 Lewis Structure (Carbon Tetrachloride) YouTube
Lewis Structures CCl4 YouTube
Lewis Dot Diagram For Ccl4 Wiring Site Resource
CCl4 Lewis structure in four simple steps What's Insight
Drawing Lewis Structure for CCl4 and Determining Geometries and Bond
CCl4 Lewis Structure ,Valence Electrons ,Formal Charge ,Polar or Nonpolar
20+ Ccl4 Molecular Geometry Name Full GM
CCl4 Lewis Structure Science Trends
The Following Procedure Will Give You The Correct Lewis Structure For Any Molecule Or Polyatomic Ion That Has One Central Atom.
Web How To Draw A Lewis Structure For Ccl4?
The Lewis Structure For Ccl4 Is A Commonly Tested Lewis Struc.
There Are 3 Lone Pairs On All The Four Chlorine Atoms (Cl).
Related Post: