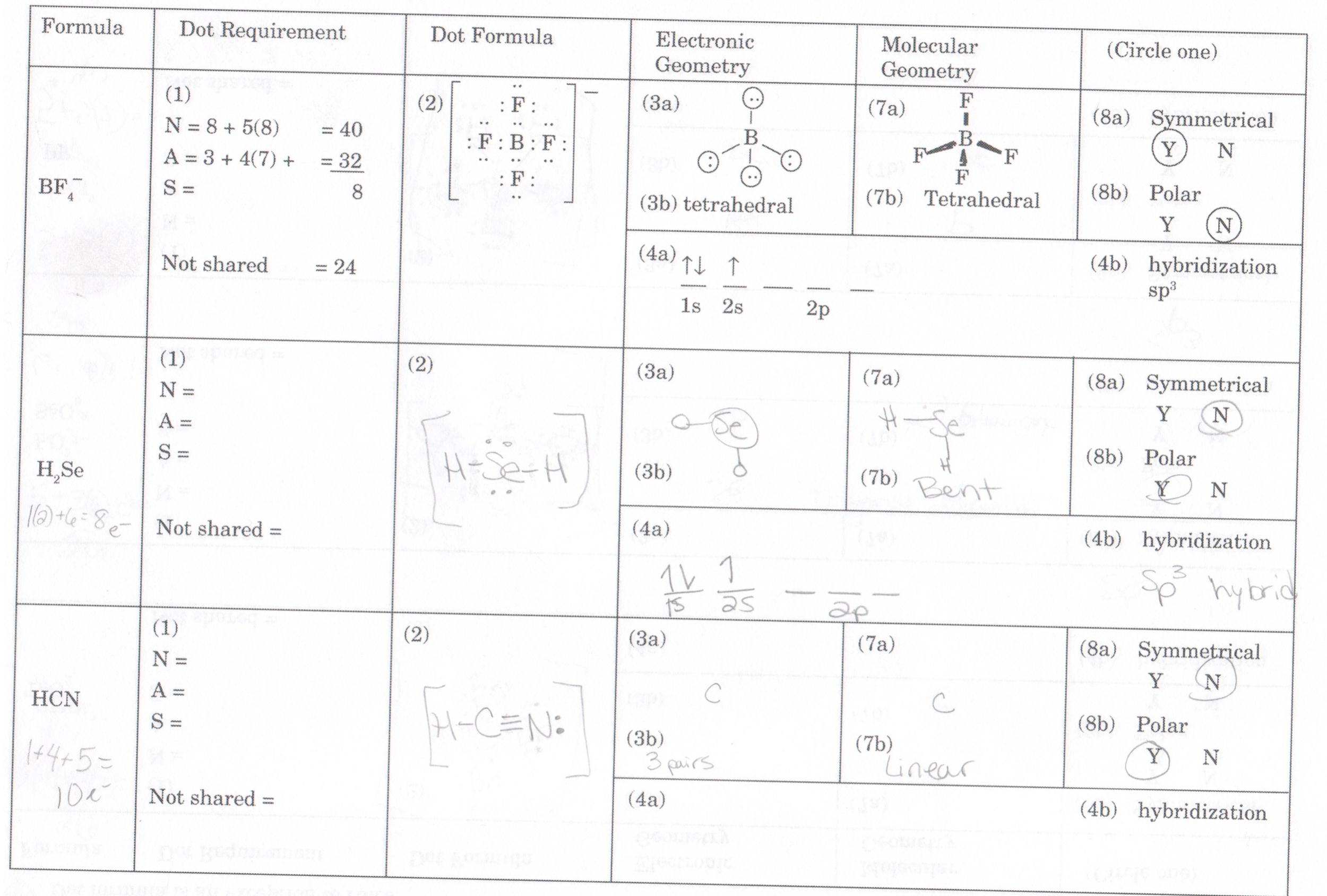
Draw The Lewis Structure For Chclo
Draw The Lewis Structure For Chclo - Shared pairs of electrons are drawn as lines between atoms, while lone pairs of. Web draw a plausible lewis electron structure for a compound with the molecular formula cl 3 po. ∠cl −c −o and ∠h − c − o are roughly 120 ∘. The lewis structure of chclo consists of one carbon atom (c), one hydrogen atom (h), one chlorine atom (cl), and one oxygen atom (o). Web draw the lewis structure for chclo. Use these steps to correctly draw the chclo lewis structure: Web draw lewis structures depicting the bonding in simple molecules. Draw the molecule by placing the atoms on the grid and connecting them with bonds. #1 first draw a rough sketch. Lewis structures are representations of molecules that include not only what atoms are present in the. Web write lewis symbols for neutral atoms and ions. Formyl chloride has 3 regions of electron density around the carbon, and a formal pπ −pπ c − o bond. Draw lewis structures depicting the bonding in simple molecules. First, lets find the how many valence electrons chlorate has: Draw the molecule by placing the atoms on the grid and connecting them with bonds. Draw lewis structures for covalent compounds. Compute formal charges for atoms in any lewis structure. #2 mark lone pairs on the atoms. Determine the total number of valence electrons in one hypochlorite molecule. The lewis structure of chclo consists of one carbon atom (c), one hydrogen atom (h), one chlorine atom (cl), and one oxygen atom (o). Understand the proper use of the octet rule to predict. Draw a three dimensional representation of the molecule. Web your solution’s ready to go! Draw lewis structures depicting the bonding in simple molecules. It's the same as ch2o (formaldehyde / methanal), but with a. Web draw the lewis structure for chclo. Web write lewis symbols for neutral atoms and ions. Include all lone pairs of electrons. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of. Determine the total number of valence electrons in one hypochlorite molecule. Web draw a plausible lewis electron structure for a compound with the molecular formula cl 3 po. Web steps to draw a lewis structure of hypochlorite (clo) step 1: Draw a three dimensional representation of the molecule. Web your solution’s ready to go! Web this widget gets the lewis structure of chemical compounds. Draw a three dimensional representation of the molecule. $7 + 6 + 1 = 14$ total electrons needed for octets/doublets:. Include all lone pairs of electrons. Web draw lewis structures depicting the bonding in simple molecules. The lewis structure of chclo consists of one carbon atom (c), one hydrogen atom (h), one chlorine atom (cl), and one oxygen atom (o). Lewis structures are representations of molecules that include not only what atoms are present in the. Web your solution’s ready to go! Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web draw lewis structures depicting the bonding in simple molecules. Compute formal charges for atoms in any lewis structure. #3 calculate and mark formal charges on the. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of. Web draw the lewis structure for chclo. Determine the total number of valence electrons in one hypochlorite molecule. Lewis structures are representations of molecules that include not only what atoms are present in the. Web draw a plausible lewis electron structure for a compound with the molecular formula cl 3 po. The chlorine atom has seven valence. First, lets find the how many valence electrons chlorate has: Understand the proper use of the octet rule to predict. #1 first draw a rough sketch. #1 first draw a rough sketch. Draw lewis structures for covalent compounds. Draw the molecule by placing the atoms on the grid and connecting them with bonds. Lewis structures are representations of molecules that include not only what atoms are present in the. Formyl chloride has 3 regions of electron density around the carbon, and a formal pπ −pπ c. Draw lewis structures depicting the bonding in simple molecules. Use formal charges to identify the most reasonable. Compute formal charges for atoms in any lewis structure. Draw lewis structures for covalent compounds. It displays the bonds that exist between a molecule's atoms, as well as the unpaired electrons that are. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of. It's the same as ch2o (formaldehyde / methanal), but with a. #3 calculate and mark formal charges on the. Draw lewis structures for covalent compounds. Determine the total number of valence electrons in one hypochlorite molecule. Web your solution’s ready to go! Determine the total number of valence electrons in one hypochlorite molecule. It's the same as ch2o (formaldehyde / methanal), but with a. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Draw lewis structures depicting the bonding in simple molecules. Draw lewis structures depicting the bonding in simple molecules. Web the properly way to determine the lewis structure, based on this example, is: #1 first draw a rough sketch. Web steps to draw a lewis structure of hypochlorite (clo) step 1: Web draw the lewis structure for chclo. Include all lone pairs of electrons. ∠cl −c −o and ∠h − c − o are roughly 120 ∘. Formyl chloride has 3 regions of electron density around the carbon, and a formal pπ −pπ c − o bond. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of. #3 calculate and mark formal charges on the. Use these steps to correctly draw the chclo lewis structure: Web write lewis symbols for neutral atoms and ions. It displays the bonds that exist between a molecule's atoms, as well as the unpaired electrons that are. It's the same as ch2o (formaldehyde / methanal), but with a. Lewis structures are representations of molecules that include not only what atoms are present in the. Web draw a plausible lewis electron structure for a compound with the molecular formula cl 3 po. Determine the total number of valence electrons in one hypochlorite molecule. First, lets find the how many valence electrons chlorate has: $7 + 6 + 1 = 14$ total electrons needed for octets/doublets:. #2 mark lone pairs on the atoms. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.Determine whether CHClO is an ionic or molecular compound and draw an
Chclo Lewis Structure What The Molecular Shape Of Chclo Clutch Prep
Chclo Lewis Structure What The Molecular Shape Of Chclo Clutch Prep
Draw The Lewis Structure For Chclo
Chclo Lewis Structure What The Molecular Shape Of Chclo Clutch Prep
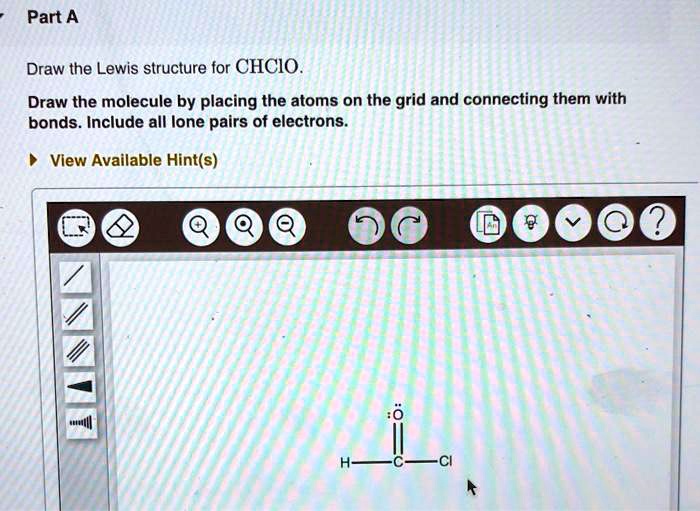
SOLVED Draw the Lewis structure for CHClO. Draw the molecule by
Chclo Lewis Structure What The Molecular Shape Of Chclo Clutch Prep
Solved Draw the electrondot structure for CHClO. Draw the
Draw The Lewis Structure For Chclo Drawing.rjuuc.edu.np
CHClO Lewis Structure How to Draw the Lewis Structure for CHClO YouTube
Draw Lewis Structures Depicting The Bonding In Simple Molecules.
The Lewis Structure Of Chclo Consists Of One Carbon Atom (C), One Hydrogen Atom (H), One Chlorine Atom (Cl), And One Oxygen Atom (O).
Web Steps To Draw A Lewis Structure Of Hypochlorite (Clo) Step 1:
Web Your Solution’s Ready To Go!
Related Post: