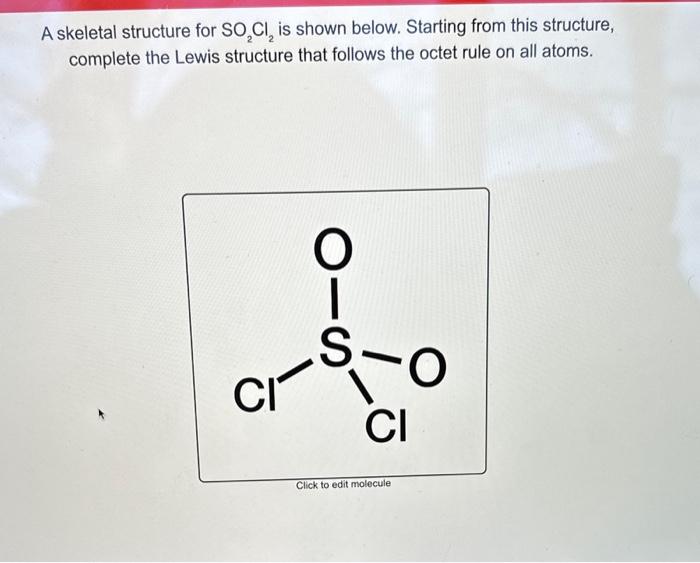
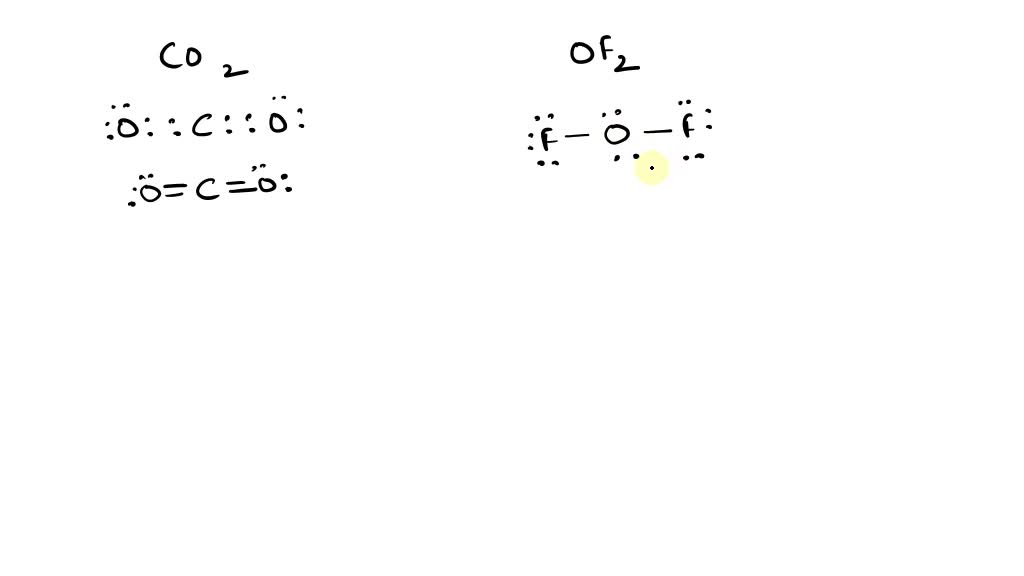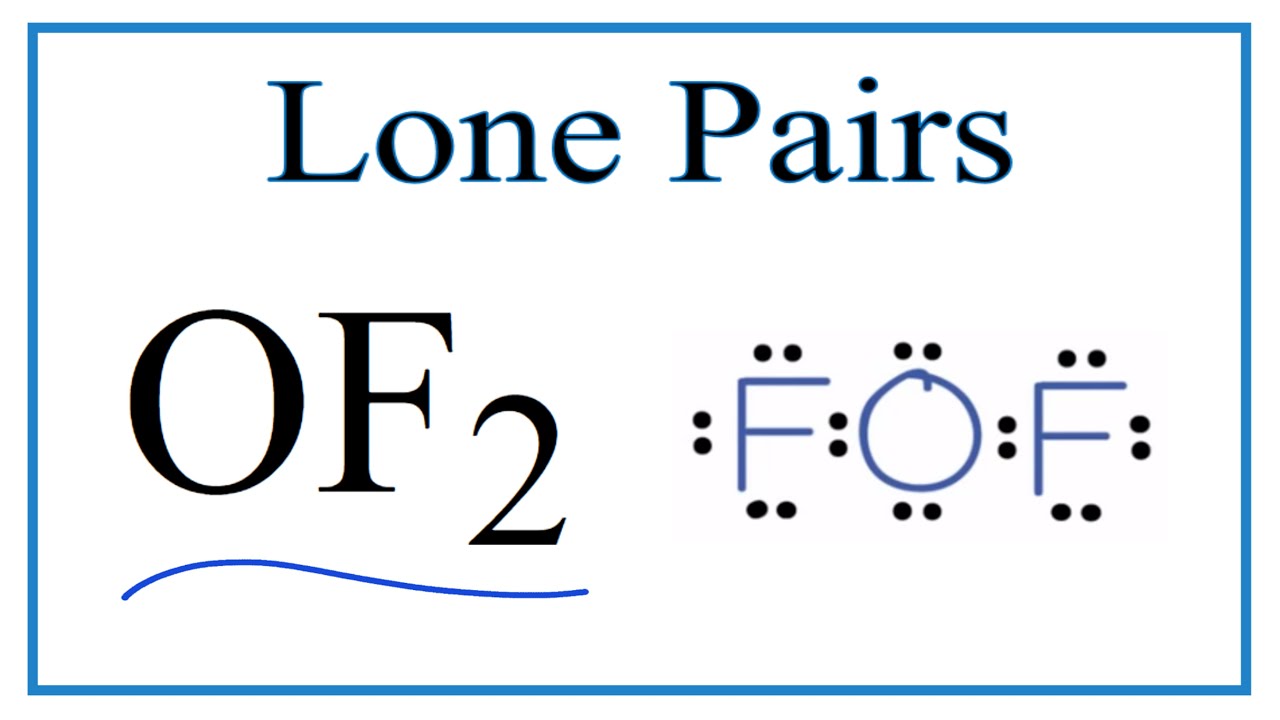Draw The Lewis Structure For Of2
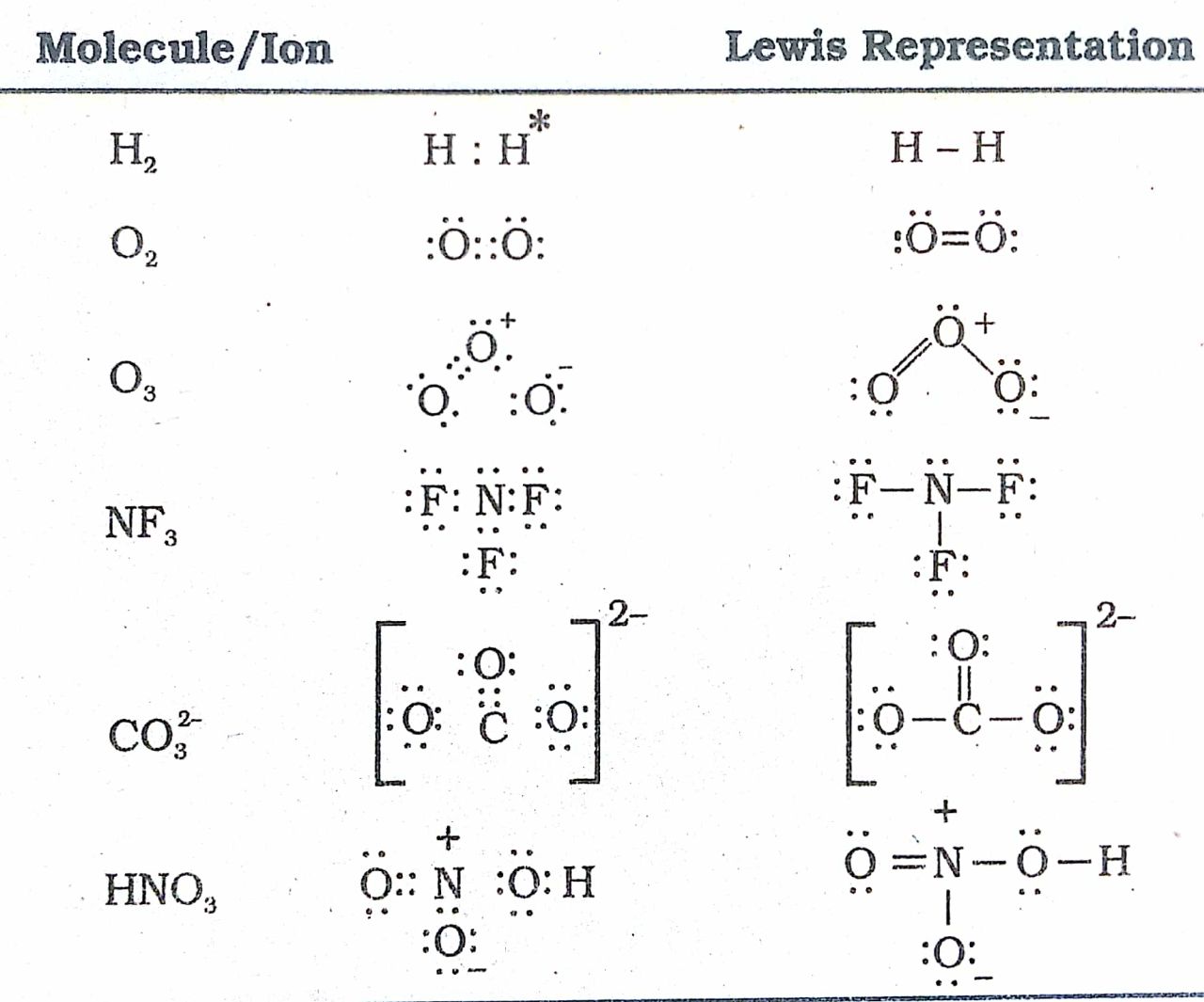
Draw The Lewis Structure For Of2 - Web drawing lewis structures for molecules with one central atom: Oxygen difluoride or of2 is a chemical compound formed by the reaction between halogen fluorine and dilute aqueous solution of naoh ( sodium hydroxide ). Find the total valence electrons in of2 molecule. In order to find the total valence electrons in of2 (oxygen difluoride) molecule, first of all you should know the valence electrons present in oxygen atom as well as fluorine atom. Here, the given molecule is of2 (oxygen difluoride). Count total valence electrons present in of2 molecule. There are three lone pairs on each fluorine atom, and two lone pairs on the oxygen atom. Web in the lewis structure of of2, both fluorine atoms share a single bond with the oxygen. #3 calculate and mark formal charges on the atoms, if required. Web learn how to draw a lewis structure to show the bonding and valence electrons in a molecule. See why lewis structures are important. Web how to draw lewis structure for of2? Web 2.8k views 3 years ago lewis structure (chemistry) how to draw lewis structure for of2 oxygen difluoride lewis structure: Oxygen difluoride or of2 is a chemical compound formed by the reaction between halogen fluorine and dilute aqueous solution of naoh ( sodium hydroxide ). A video explanation of how to draw the lewis dot structure for oxygen difluoride, along with information about the compound. Oxygen is in the center, and fluorine atoms surround it, forming a. Web we draw lewis structures to predict: #4 calculate formal charge and check stability. #1 first draw a rough sketch. Web draw the correct lewis structure of of2 and then use it to answer the questions below. The equation for the preparation of oxygen difluoride: Web hey guys,in this video we are going to learn about the lewis structure of of2. Web this widget gets the lewis structure of chemical compounds. There are three lone pairs on each fluorine atom, and two lone pairs on the oxygen atom. In order to draw the lewis structure of of2, first of all you have to find the total number of valence electrons present in the of2 molecule. Oxygen difluoride or of2 is a chemical compound formed by the reaction between halogen fluorine and dilute aqueous solution of naoh ( sodium hydroxide ). Web what is the lewis structure of of2? Web the of2 lewis structure is a diagram that shows the arrangement of atoms and electrons in the molecule. [ select] b) how many lone pairs of electrons are present on the central atom in the lewis structure? A video explanation of how to draw the lewis dot structure for oxygen difluoride, along with information about the compound. Web of2 lewis structure, molecular geometry, hybridization, polarity, and mo diagram. 8 + (6 × 7) = 50. #3 calculate and mark formal charges on the atoms, if required. 13k views 11 years ago chemistry lewis dot structures. Calculate the total number of valence electrons. 8 + (2 × 7) = 22 xef 6: The equation for the preparation of oxygen difluoride: Oxygen belongs to group 16th and fluorine belongs to group 17th of the periodic table. In order to find the total valence electrons in of2 (oxygen difluoride) molecule, first of all you should know the valence electrons present in oxygen atom as well. Let’s discuss each step in more detail. Find the total valence electrons in of2 molecule. #1 first draw a rough sketch. See why lewis structures are important. Count total valence electrons present in of2 molecule. 8 + (2 × 7) = 22 xef 6: Web learn how to draw a lewis structure to show the bonding and valence electrons in a molecule. Web we draw lewis structures to predict: There are three lone pairs on each fluorine atom, and two lone pairs on the oxygen atom. 8 + (6 × 7) = 50. Find more chemistry widgets in wolfram|alpha. First step is to find the no. Oxygen brings 6 electrons, each fluorine brings 7 electrons, that's 20 electrons total. #4 calculate formal charge and check stability. Here, the given molecule is of2 (oxygen difluoride). Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web of2, aka oxygen difluoride, is a molecular compound. Web how to draw lewis structure for of2? Web what is the lewis structure of of2? #4 calculate formal charge and check stability. Here, the given molecule is of2 (oxygen difluoride). #1 first draw a rough sketch. Oxygen belongs to group 16th and fluorine belongs to group 17th of the periodic table. #2 mark lone pairs on the atoms. In order to find the total valence electrons in of2 (oxygen difluoride) molecule, first of all you should know the valence electrons present in. 8 + (2 × 7) = 22 xef 6: It follows the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons. 13k views 11 years ago chemistry lewis dot structures. Following are the steps to follow to draw the lewis structure of of2 molecule. A) how many. Here, the given molecule is of2 (oxygen difluoride). Web drawing lewis structures for molecules with one central atom: Web draw the correct lewis structure of of2 and then use it to answer the questions below. Find more chemistry widgets in wolfram|alpha. Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Oxygen brings 6 electrons, each fluorine brings 7 electrons, that's 20 electrons total. Following are the steps to follow to draw the lewis structure of of2 molecule. Web of2 lewis structure, molecular geometry, hybridization, polarity, and mo diagram. In order to find the total valence electrons in of2 (oxygen difluoride) molecule, first of all you should know the valence electrons. In order to draw the lewis structure of of2, first of all you have to find the total number of valence electrons present in the of2 molecule. Lone pairs, unpaired electrons, and. Web steps of drawing of2 lewis structure. Draw a skeleton joining the atoms by single bonds. Use these steps to correctly draw the of 2 lewis structure: Find the total valence electrons in of2 molecule. #4 calculate formal charge and check stability. Web draw the correct lewis structure of of2 and then use it to answer the questions below. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Find more chemistry widgets in wolfram|alpha. 13k views 11 years ago chemistry lewis dot structures. Web learn how to draw a lewis structure to show the bonding and valence electrons in a molecule. A video explanation of how to draw the lewis dot structure for oxygen difluoride, along with information about the compound. • how to draw lewis structure for pocl3. The lewis structure of of2, oxygen difluoride, consists of one oxygen atom (o) bonded with two fluorine atoms (f) through covalent bonds. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions).Solved Draw the correct Lewis structure of OF2 and then use
Easy Steps to Draw Lewis Structure
5 Easy Steps for OF2 lewis Structure,Hybridization (Solved)
Draw the Lewis structures of the given molecules. Include lone pairs on
Solved Draw the Lewis structure of OF2 and then determine
Draw The Lewis Structure Of Of2
Drawing Lewis Dot Structures For Covalent Compounds Youtube
OF2 Lewis Structure How to Draw the Lewis Structure for OF2 YouTube
SOLVEDConsider the molecules CO2 and OF2 a. Draw a valid Lewis
Number of Lone Pairs and Bonding Pairs for OF2 (Oxygen difluoride
Web Of2, Aka Oxygen Difluoride, Is A Molecular Compound.
Web A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
Oxygen Difluoride Or Of2 Is A Chemical Compound Formed By The Reaction Between Halogen Fluorine And Dilute Aqueous Solution Of Naoh ( Sodium Hydroxide ).
There Are Three Lone Pairs On Each Fluorine Atom, And Two Lone Pairs On The Oxygen Atom.
Related Post: