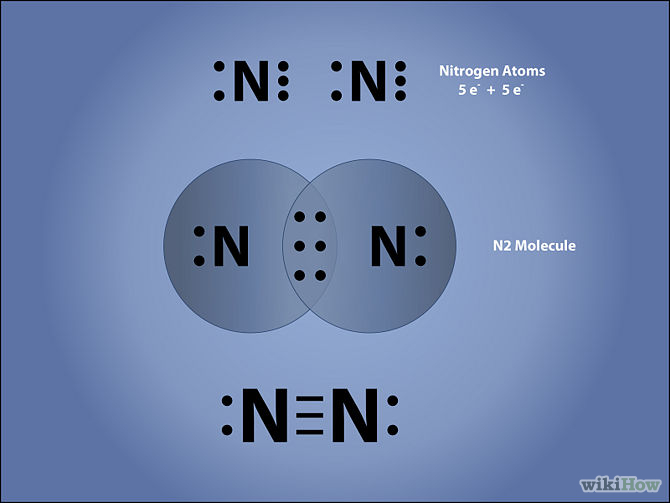
Draw The Lewis Structure Of N2
Draw The Lewis Structure Of N2 - Web what is the lewis structure of n 2? To properly draw the n 2 lewis structure, follow these steps: * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Web the n 2 lewis structure has a triple bond between two nitrogen atoms. And both the nitrogen atoms have one lone pair on it. Sum the valence electrons from all the atoms. Also, for the structure to be correct, it must follow the octet rule (eight electrons per atom). #1 draw a rough sketch of the structure. #3 indicate formal charges on the atoms, if necessary. I also go over the shape and bond angles. Determine the total number of valence electrons in the molecule by adding the valence electrons of each nitrogen atom. Web using the periodic table to draw lewis dot structures; Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. #2 next, indicate lone pairs on the atoms. Web to draw the n2 lewis structure, you can follow these steps: Web the n 2 lewis structure has a triple bond between two nitrogen atoms. Drawing lewis dot structures for polyatomic ions; How to draw a lewis structure; The structure consists of a triple bond between the two nitrogen atoms and lone pairs on one of the nitrogen atoms. See the big list of lewis structures. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Examples for drawing lewis structures for covalent bonds; See the big list of lewis structures. The n 2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. Web in the lewis structure of the n2 molecule, there is a formation of a triple covalent bond represented by three lines between two atoms of nitrogen. Web i quickly take you through how to draw the lewis structure of n2 (dinitrogen). Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. In the case of n2, each nitrogen atom has five valence electrons, so the total number of valence electrons is 10. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. #2 next, indicate lone pairs on the atoms. Web in short, these are the steps you need to follow for drawing a lewis structure: How to draw a lewis structure; Nitrogen gas (diatomic nitrogen) watch on. #5 repeat step 4 if necessary, until all charges are minimized. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. #5 repeat step 4 if necessary, until all charges are minimized. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Determine the total number of valence electrons in the molecule by adding the valence electrons of each nitrogen atom. The lewis structure of n 2 has nitrogen. Web in this video i will show you the fundamentals on how to draw a lewis dot structure as while showing you how to draw the lewis dot structure for n2. Practice with drawing lewis structures Web what is the lewis structure of n 2? There are many things to learn when we draw n 2 lewis structure. Nitrogen gas. To draw the lewis structure of n2, first to find out the valance electron of nitrogen. Practice with drawing lewis structures Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. #1 draw a rough sketch of the structure. The n 2 molecule is diatomic, meaning that two. And both the nitrogen atoms have one lone pair on it. The lewis structure of n 2 has nitrogen bound to the other nitrogen by a triple bond, with a one pair of electrons on each nitrogen atom. Practice with drawing lewis structures How to draw a lewis structure; Web here is the electron dot structure for a single #n#. Web what is the lewis structure of n 2? Sum the valence electrons from all the atoms. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. #2 next, indicate lone pairs on the atoms. #4 minimize formal charges by converting lone pairs of the atoms. There are many things to learn when we draw n 2 lewis structure. Drawing lewis dot structures for polyatomic ions; What is this molecule and what is it used for? #1 draw a rough sketch of the structure. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. How to draw a lewis structure; Web what is the lewis structure of n 2? Web nitrogen is a diatomic molecule and contains only two nitrogen atoms. To draw the lewis structure of n2, first to find out the valance electron of nitrogen. Web here is the electron dot structure for a single #n# atom: Let’s draw and understand this lewis dot structure step by step. #3 indicate formal charges on the atoms, if necessary. Examples for drawing lewis structures for covalent bonds; Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Shared pairs of electrons are drawn as lines between atoms, while lone pairs. Drawing lewis dot structures for polyatomic ions; Let’s draw and understand this lewis dot structure step by step. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web using the periodic table to draw lewis dot structures; Examples for drawing lewis structures for covalent bonds; Web in the lewis structure of the n2 molecule, there is a formation of a triple covalent bond represented by three lines between two atoms of nitrogen. To draw the lewis structure of n2, first to find out the valance electron of nitrogen. #1 draw a rough sketch of the structure. #5 repeat step 4 if necessary, until all charges are minimized. Lewis structure of n 2 molecule contains a triple bond and each nitrogen atom has one lone pair. Examples for drawing lewis structures for covalent bonds; Web using the periodic table to draw lewis dot structures; See the big list of lewis structures. I also go over the shape and bond angles. Web nitrogen is a diatomic molecule and contains only two nitrogen atoms. How do we find the valance electron of an atom? Web what is the lewis structure of n 2? Sum the valence electrons from all the atoms. How to draw a lewis structure; Web the n 2 lewis structure has a triple bond between two nitrogen atoms. Also, for the structure to be correct, it must follow the octet rule (eight electrons per atom).N2 Structure
Lewis Dot structure for N2? PHOTO ABOVE Is not D I got it wrong
N2 Lewis Structure How To Draw The Lewis Structure For N2
N2 Structure
N2 Lewis Structure N2 Lewis Dot Structure Nitrogen Gas Lewis
N2 Lewis Diagram
How To Draw Electron Dot Diagrams
N2 Lewis Structure
Lewis Dot Structure For N2 Draw Easy
Lewis structure of N2 (Nitrogen gas) YouTube
#2 Next, Indicate Lone Pairs On The Atoms.
Web I Quickly Take You Through How To Draw The Lewis Structure Of N2 (Dinitrogen).
In The Case Of N2, Each Nitrogen Atom Has Five Valence Electrons, So The Total Number Of Valence Electrons Is 10.
And Both The Nitrogen Atoms Have One Lone Pair On It.
Related Post: