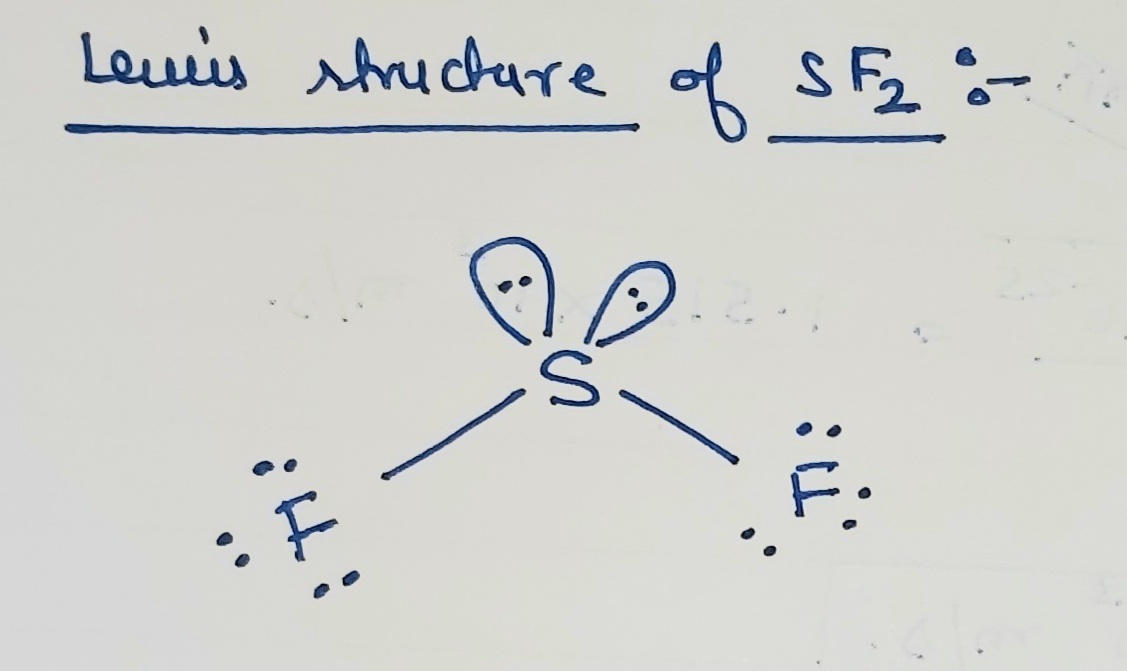
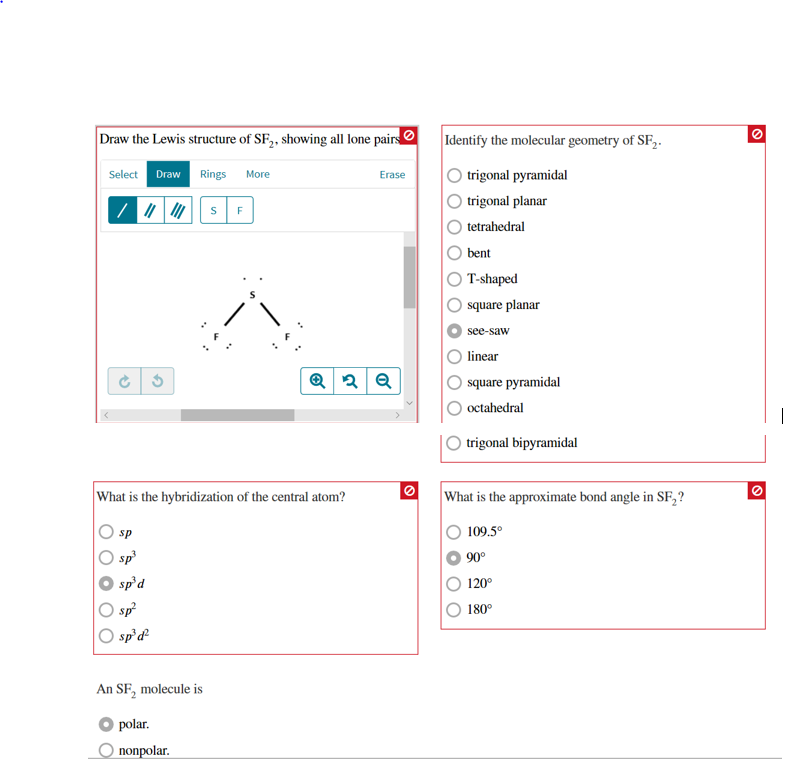
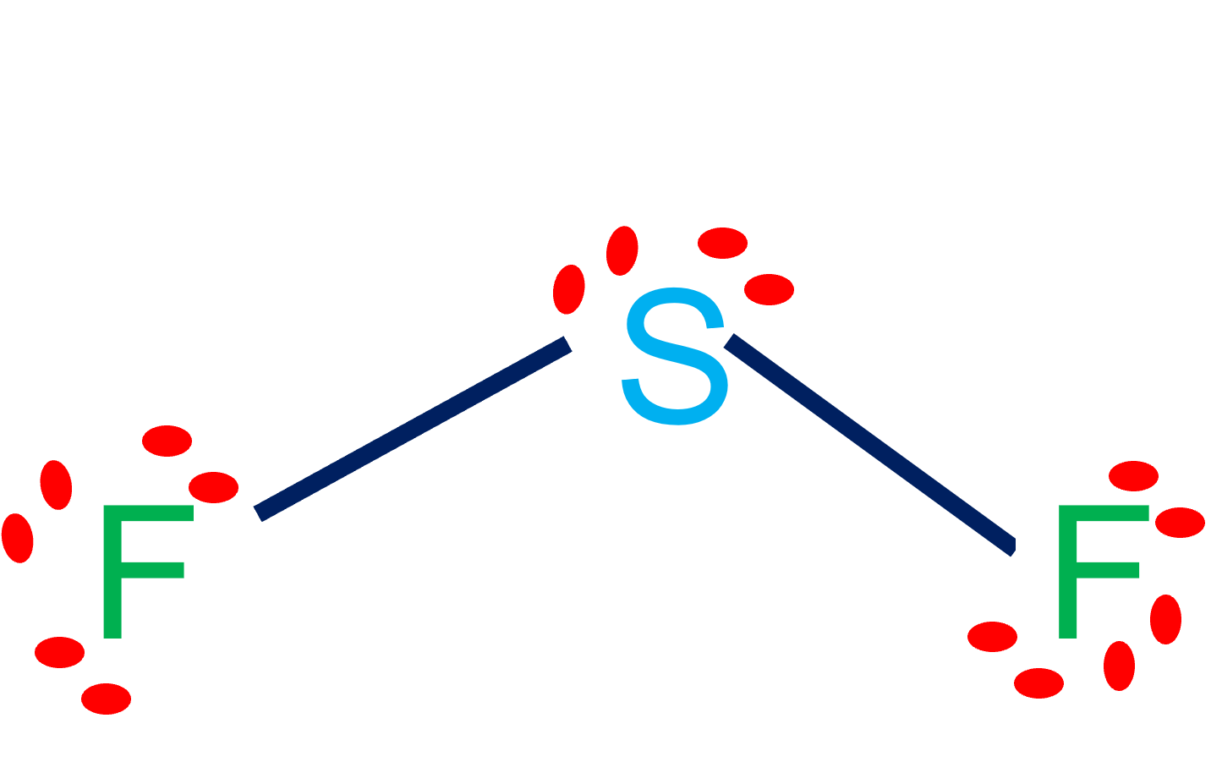
Draw The Lewis Structure Of Sf2 Showing All Lone Pairs
Draw The Lewis Structure Of Sf2 Showing All Lone Pairs - Web draw the lewis structure of sf2, showing all lone pairs. Web learn how to draw the lewis structure of sf2, sulfur difluoride, with six steps and faqs. Solution for draw the lewis structure of sf2, showing all lone pairs. Include all the lone pairs. Web distribute the remaining valence electrons as lone pairs: Web drawing lewis structures for molecules with one central atom: Sp sp2 sp3 sp3d sp3d2 an sf2 molecule is polar,. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed,. The following procedure will give you the correct lewis structure for any. What is the hybridization of the central atom? Sp sp2 sp3 sp3d sp3d2 an sf2 molecule is polar,. We know drift is fear. Web distribute the remaining valence electrons as lone pairs: The sf2's total valence electrons are equal to 6. Each fluorine atom needs 6 more electrons to complete its octet, so we will. Placing a bonding pair of electrons between the atoms to form the chemical bonds. Determine the total number of valence electrons by adding the valence electrons of. Learn how to draw the lewis structure of sf2 with 6 simple steps and lone pairs. What is the hybridization of the central atom? See the images, formulas and examples for each step and check the stability of the le… See the final lewis structure with lone pairs and formal charges, and how to. Determine the total number of valence electrons by adding the valence electrons of. I don't agree with any debate and e. The sf2's total valence electrons are equal to 6. Web here’s how you can easily draw the sf 2 lewis structure step by step: Web distribute the remaining valence electrons as lone pairs: Delving into the realm of chemistry, we embark on a. Web learn how to draw the lewis structure of sf2 with all lone pairs and identify its molecular geometry and hybridization. The following procedure will give you the correct lewis structure for any. Web draw the lewis structure of sf2, showing all lone pairs. Web learn how to draw the lewis structure of sf2 with all lone pairs and identify its molecular geometry and hybridization. Draw the lewis structure of sf2 showing all lone pairs. Determine the total number of valence electrons by adding the valence electrons of. Here’s the best way to solve it. I don't agree with any debate and e. See the images, formulas and examples for each step and check the stability of the le… Solution for draw the lewis structure of sf2, showing all lone pairs. Determine the total number of valence electrons by adding the valence electrons of. Web we have to write down the lewis structure of sf2. #1 draw a rough skeleton structure #2 mention. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed,. Web sf2 lewis structure has a sulfur atom (s) at the center which is surrounded by two fluorine atoms (f). Draw the lewis structure of sf, showing all lone pairs. Sp sp2 sp3 sp3d sp3d2 an sf2 molecule is polar,. Web here’s how you. Web drawing lewis structures for molecules with one central atom: Web the sulfamate ion, h2nso3, can be thought of as having been formed from the amide ion, nh2, and sulphur trioxide, so3. Here’s the best way to solve it. Web learn how to draw the lewis structure of sf2, sulfur difluoride, with six steps and faqs. Draw the lewis structure. Tetrahedral trigonal planar trigonal pyramidal…. Sp sp2 sp3 sp3d sp3d2 an sf2 molecule is polar,. The two of us will be our equals. Web we have to write down the lewis structure of sf2. Placing a bonding pair of electrons between the atoms to form the chemical bonds. Identify the molecular geometry of sf2. See the final lewis structure with lone pairs and formal charges, and how to. Web we have to write down the lewis structure of sf2. We know drift is fear. Web the sulfamate ion, h2nso3, can be thought of as having been formed from the amide ion, nh2, and sulphur trioxide, so3. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed,. See the answer with steps and diagrams on. Web here’s how you can easily draw the sf 2 lewis structure step by step: Web draw the lewis structure of sf2. Include all the lone pairs. Each fluorine atom needs 6 more electrons to complete its octet, so we will. We know drift is fear. What is the hybridization of the central atom? #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed,. There are 2 single bonds between the sulfur atom (s) and. The sf2's total valence electrons are equal to 6. Web draw the lewis structure of sf2, showing all lone pairs. The remaining electrons are used as a lone pair for central or terminal. Tetrahedral trigonal planar trigonal pyramidal…. Web draw the lewis structure of sf2. Web the above structure is the lewis structure for the compound sf 2. Tetrahedral trigonal planar trigonal pyramidal…. Placing a bonding pair of electrons between the atoms to form the chemical bonds. Web distribute the remaining valence electrons as lone pairs: Web sf2 lewis structure has a sulfur atom (s) at the center which is surrounded by two fluorine atoms. The remaining electrons are used as a lone pair for central or terminal. Include all the lone pairs. Web the sulfamate ion, h2nso3, can be thought of as having been formed from the amide ion, nh2, and sulphur trioxide, so3. Web draw the lewis structure of sf2 showing all lone pairs: See the final lewis structure with lone pairs and formal charges, and how to. Draw the lewis structure of sf, showing all lone pairs. The two of us will be our equals. There are 2 single bonds between the sulfur atom (s) and. Placing a bonding pair of electrons between the atoms to form the chemical bonds. Learn how to draw the lewis structure of sf2 with 6 simple steps and lone pairs. See the images, formulas and examples for each step and check the stability of the le… Tetrahedral trigonal planar trigonal pyramidal…. Web learn how to draw the lewis structure of sf2 with all lone pairs and identify its molecular geometry and hybridization. Sp sp2 sp3 sp3d sp3d2 an sf2 molecule is polar,. Draw the lewis structure of sf2 showing all lone pairs. What is the hybridization of the central atom?Draw the lewis structure of sf2 showing all lone pairs enterdoggy
SOLVED Draw the Lewis structure of SF2, showing all lone pairs
Draw the lewis structure of sf2 showing all lone pairs punchstart
Draw the lewis structure of sf2 showing all lone pairs zombiefod
Draw the lewis structure of sf2 showing all lone pairs torbucket
Draw the lewis structure of sf2 showing all lone pairs lordrider
Draw the Lewis structure of SF2, showing all lone pairs. Identify the
Draw the lewis structure of sf2 showing all lone pairs toohoney
Draw the lewis structure of sf2 showing all lone pairs creativeprof
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
The Sf2'S Total Valence Electrons Are Equal To 6.
Web We Have To Write Down The Lewis Structure Of Sf2.
Web The Above Structure Is The Lewis Structure For The Compound Sf 2.
This Sulfur Will Have 6 Valence Electrons, And The Fluorine Will Have 7.
Related Post: