Draw The Main Lewis Structure Of Nof
Draw The Main Lewis Structure Of Nof - Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and molecular geometries. #4 convert lone pairs of the atoms, and minimize formal charges. Web nof is actually onf, since nitrogen has a higher bonding capacity than both oxygen and fluorine. The central atom is typically the least electronegative, which is nitrogen. Web drawing lewis structures for molecules with one central atom: Find the total valence electrons in nof molecule. Lone pairs, unpaired electrons, and. In order to draw the lewis structure of nof, first of all you have to find the total number of valence electrons present in the nof molecule. Understand how atoms bond in nof and their unique properties and applications. #5 repeat step 4 if needed, until all charges are minimized, to get a stable. The central atom is typically the least electronegative, which is nitrogen. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 complete octet on central atom #5 calculate formal charge and check stability Web nof is a chemical formula for nitrosyl flouride. For \(\ce{pcl3}\), the electron dot diagram is as follows: To learn about lewis structures, we will start with the lewis symbol. In order to draw the lewis structure of nof, first of all you have to find the total number of valence electrons present in the nof molecule. Calculate the total number of valence electrons. Here, the given molecule is nof. Lone pairs, unpaired electrons, and. Understand how atoms bond in nof and their unique properties and applications. #2 mark lone pairs on the atoms. The nitrogen is double bonded to the oxygen atom on one side and it is single bonded to the. Can youu imagine learning how to draw lewis structures in less than 60 seconds? Understand how atoms bond in nof and their unique properties and applications. Part b draw the main lewis structure of nof. 1.2.1 lewis structure of diatomic molecules. Determine the number of bonding electrons and the number of nonbonding electrons in the structure of bef2. #4 convert lone pairs of the atoms, and minimize formal charges. For the nof structure use the periodic table to find the total number of valence electrons for. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 complete octet on central atom #5 calculate formal charge and check stability Use these steps to correctly draw the nof lewis structure: The central atom is typically the least electronegative, which is nitrogen. Web 6 steps to draw the lewis structure of nof step #1: Find the total valence electrons in nof molecule. It was introduced in 1916 by gilbert n. #2 mark lone pairs on the atoms. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Use these steps to correctly draw the nof lewis structure: Web steps of drawing nof lewis structure. Part b draw the main lewis structure of nof. Find the total valence electrons in nof molecule. The central atom is typically the least electronegative, which is nitrogen. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). #5 repeat step 4 if needed, until all charges are minimized, to get a stable. 1.2.1 lewis structure. Drawing lewis structures for bf3, pf3 and brf3; Using formal charges to determine how many bonds to make, a different perspective. Use these steps to correctly draw the nof lewis structure: The lewis structure for nof is: Calculate the total number of valence electrons. Does this ion have any other resonance structures? In order to draw the lewis structure of nof, first of all you have to find the total number of valence electrons present in the nof molecule. 1.2.1 lewis structure of diatomic molecules. Web steps of drawing nof lewis structure. Draw nonbonding electrons using the dot notation and bonding electrons as a. Web drawing the lewis structure for nof. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 complete octet on central atom #5 calculate formal charge and check stability Web this video illustrates the thinking. Web by using the following steps, you can easily draw the lewis structure of nof. Find the total valence electrons in nof molecule. Breaking the octet rule ; Draw nonbonding electrons using the dot notation and bonding electrons as a bond. #1 first draw a rough sketch. Calculate the total number of valence electrons. Web in this video, we are going to look at how to draw lewis structures for nof. The lone electron pairs on the cl atoms are omitted for clarity. In order to draw the lewis structure of nof, first of all you have to find the total number of valence electrons present in. Web explore the lewis structure of nof (nitrosyl fluoride) and discover its molecular geometry, hybridization, and polarity. #4 convert lone pairs of the atoms, and minimize formal charges. Web by using the following steps, you can easily draw the lewis structure of nof. In order to find the total valence electrons in a nof molecule, first of all you should. Web by using the following steps, you can easily draw the lewis structure of nof. 1.2.1 lewis structure of diatomic molecules. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Part b draw the main lewis structure of nof. To learn about lewis structures, we will start with the lewis symbol. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web by using the following steps, you can easily draw the lewis structure of nof. #2 mark lone pairs on the atoms. The lone electron pairs on the cl atoms are omitted for clarity. As discussed earlier atoms are. Valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). Web nof is actually onf, since nitrogen has a higher bonding capacity than both oxygen and fluorine. In the nof lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. The lewis structure for nof is: Web nof is a chemical formula for nitrosyl flouride. Web steps of drawing nof lewis structure. #5 repeat step 4 if needed, until all charges are minimized, to get a stable. The central atom is typically the least electronegative, which is nitrogen. Web in this video, we are going to look at how to draw lewis structures for nof. Find the total valence electrons in nof molecule. Web drawing the lewis structure for nof.Solved Part B Draw the main Lewis structure of NOF. Draw
SOLVED Draw the main Lewis structure of NOF . Draw nonbonding
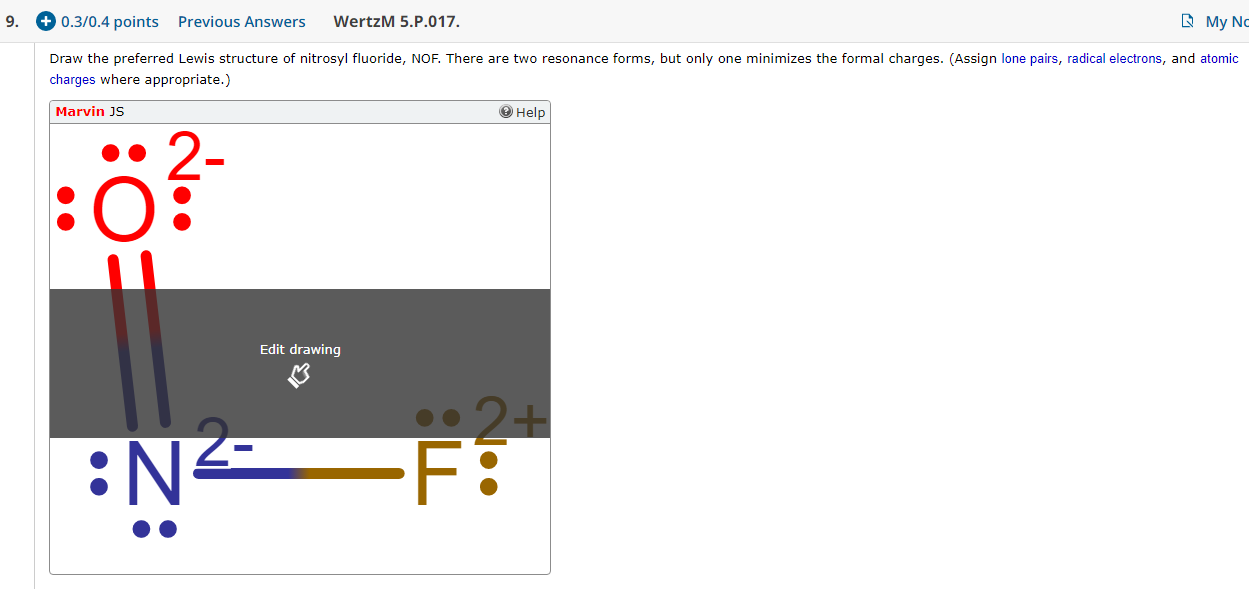
Nof Lewis Structure And Resonance Structures
How To Draw The Main Lewis Structure Of Nof learnpedia.click
NOF Lewis Structure How to Draw the Lewis Structure for NOF YouTube
NOF Lewis Structure, Geometry, Hybridization, and Polarity
Nof Lewis Structure With Charges
NOF Lewis Structure How to Draw the Lewis Structure for NOF (Nitrosyl
How To Draw The Main Lewis Structure Of Nof learnpedia.click
Draw The Main Lewis Structure Of Nof.
Check The Formal Charges To Be Sure That Each Atom Has A Formal Charge Of Zero.
Drawing Lewis Structures For Bf3, Pf3 And Brf3;
Use These Steps To Correctly Draw The Nof Lewis Structure:
Understand How Atoms Bond In Nof And Their Unique Properties And Applications.
Related Post: