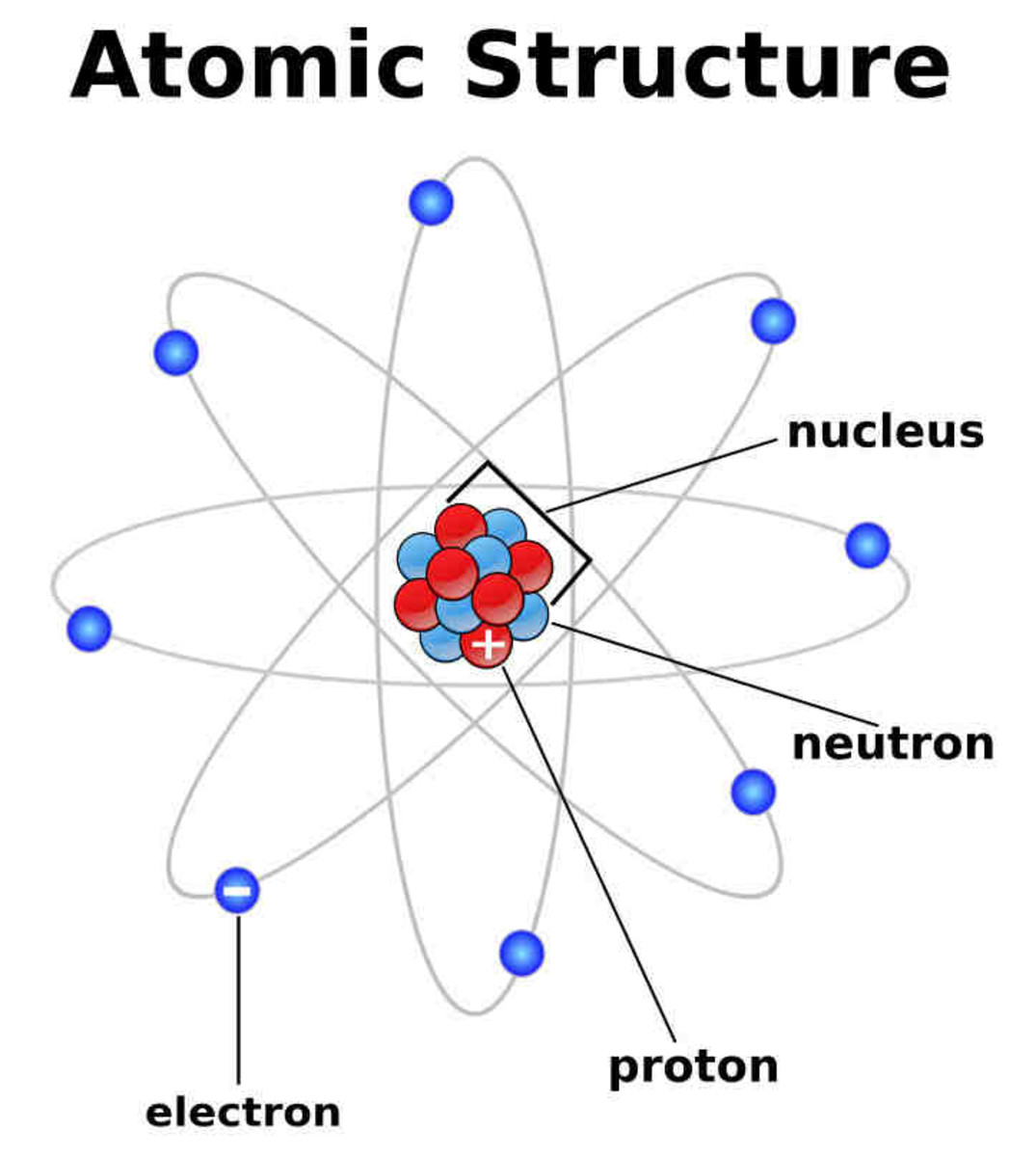
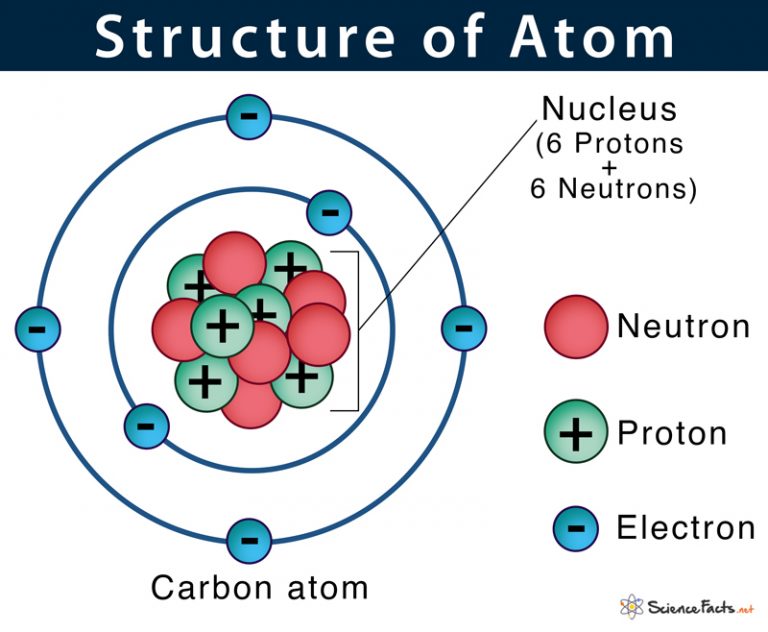
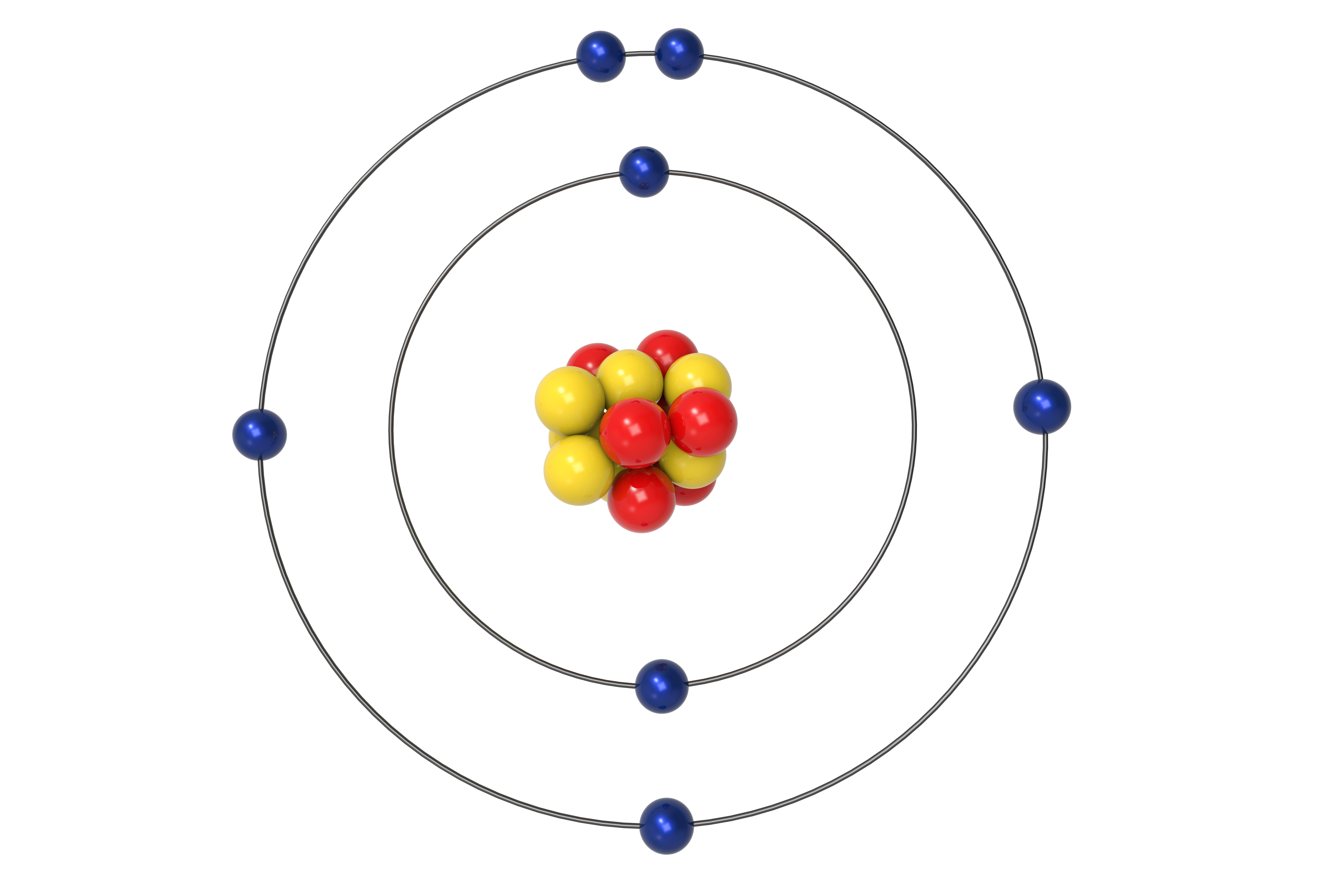
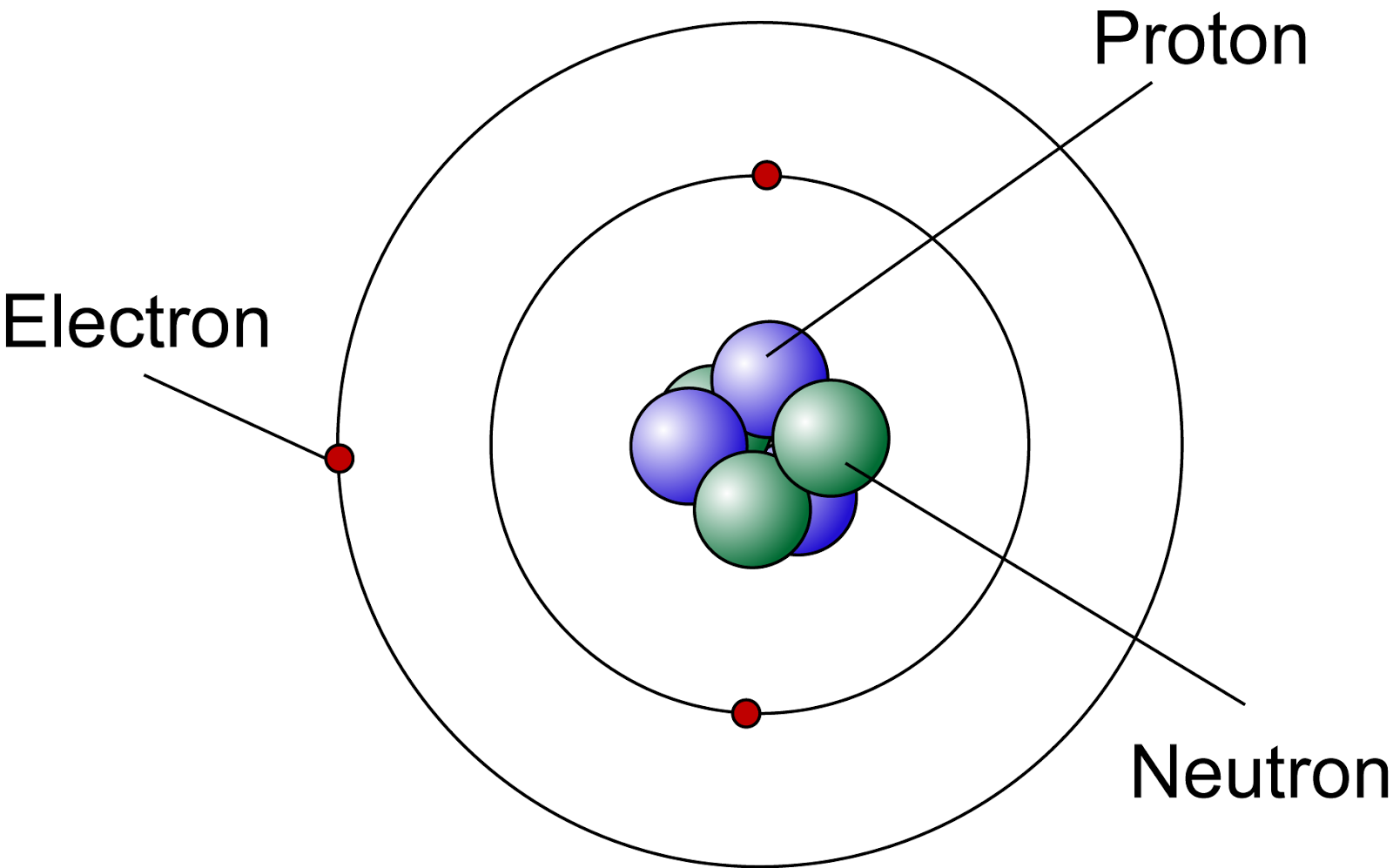
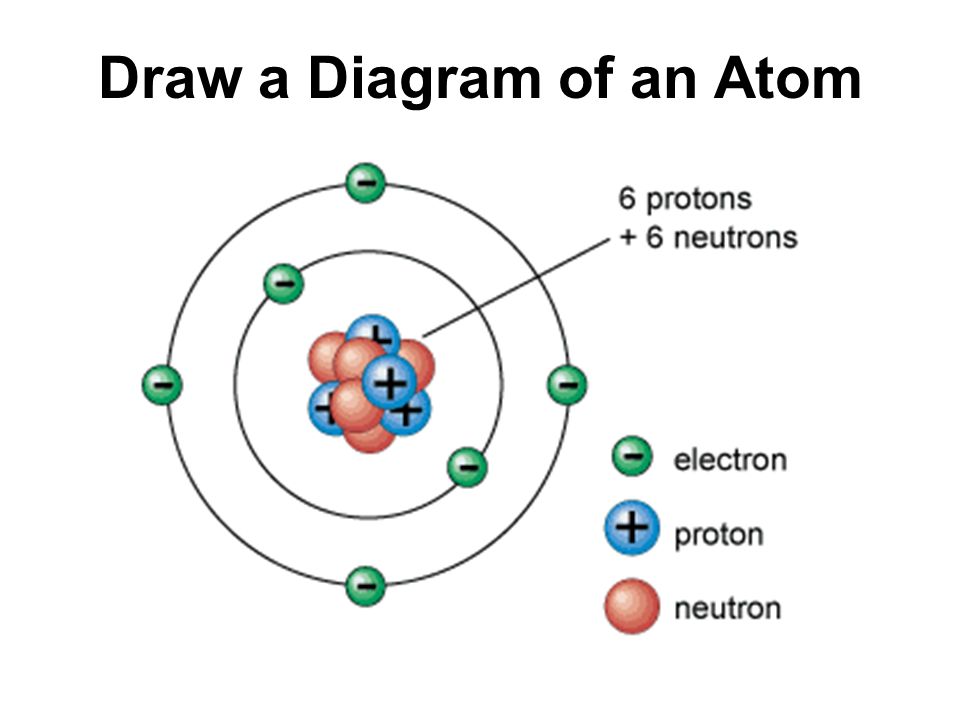
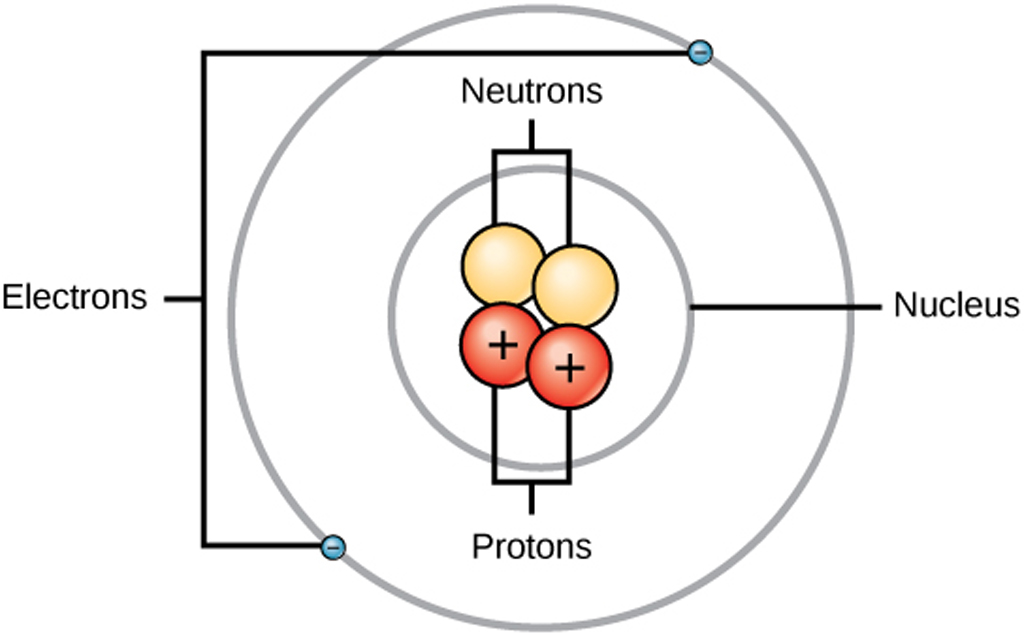
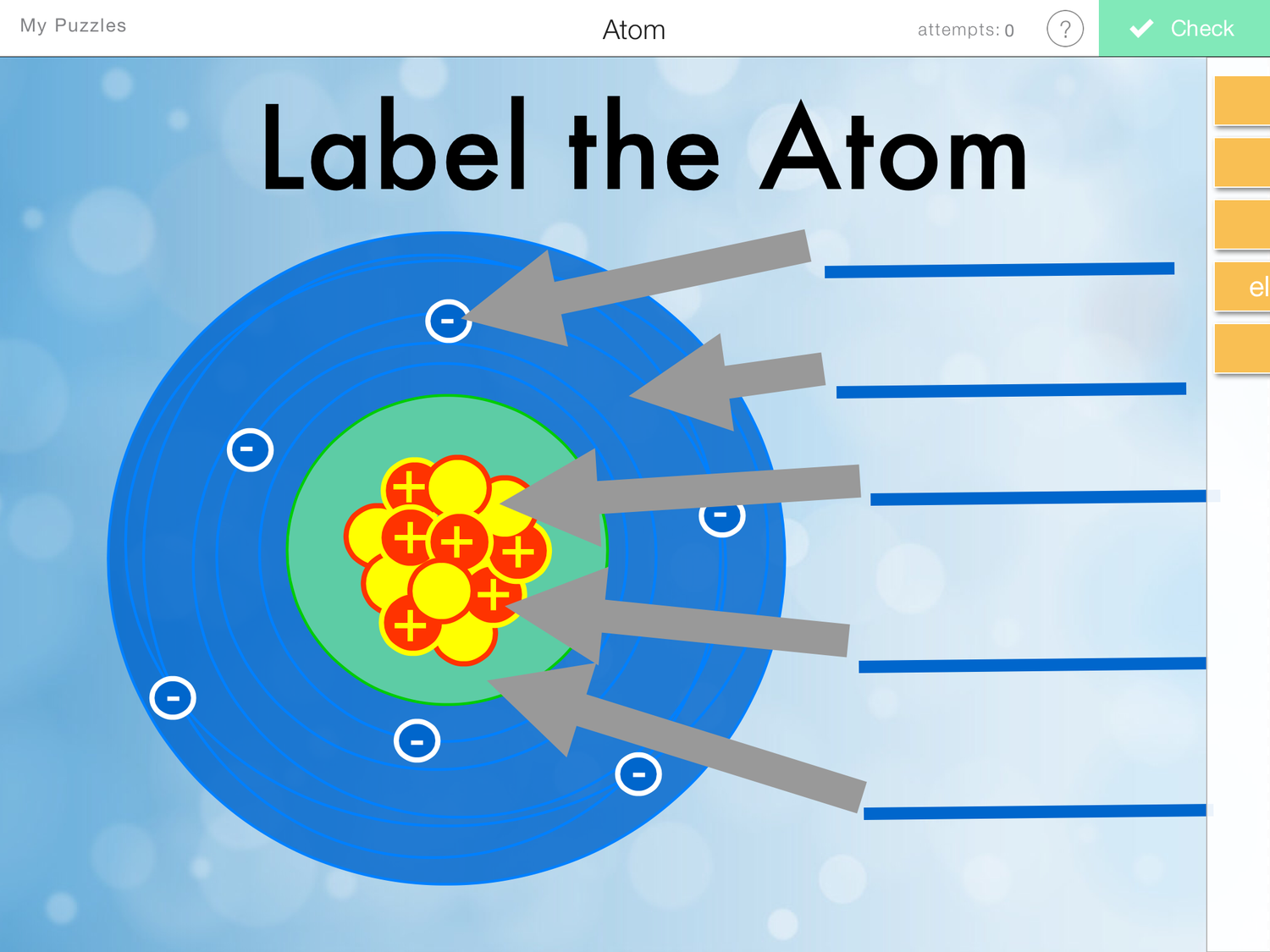
Draw The Structure Of An Atom
Draw The Structure Of An Atom - Learn how atoms are made up of protons, neutrons, and electrons. 363k views 7 years ago jefferson lab. The structure of the atom. Web drawing atomic structure requires only a simple understanding of the components of atomic structure. Web introduction to the atom. Calculate average atomic mass and isotopic abundance. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. Web rutherford’s gold foil experiment. The nucleus is tiny compared to the atom as a whole: Atoms are the smallest particle of matter than cannot be further subdivided using chemical means. 363k views 7 years ago jefferson lab. Web the three main parts of an atom are protons, neutrons, and electrons. The structure of the atom. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. In this video we cover the structure of atoms, what are subatomic particles, energy levels, and stable and reactive. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Web an atom is composed of two regions: Web rutherford’s gold foil experiment. If you understand how protons and electrons relate to one another, as well as how neutrons aid in comprising atomic mass, the rest is cake. The atom is the basic building block of matter. Atoms combine to form pure elements, compounds, and complex forms like computers and phones. Web the atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. Web in this article, the structure of an atom, you have understood what an atom is, the parts of a bit, the properties of the fundamental particles of an atom, i.e., protons, electrons, and neutrons. Web how to draw an atom! The development of modern atomic theory revealed much about the inner structure of atoms. In this video we cover the structure of atoms, what are subatomic particles, energy levels, and stable and reactive. Web what is an atom in chemistry, who discovered it, & what are they made of: The nucleus, the center of atom contain proton and neutron, and the outer portion of the atom holds electrons in its orbit around the nucleus [1]. Basic diagram of an atom most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Download complete chapter notes of structure of atom. Web the atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. Ions are those species which have a positive or a negative charge. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. The nucleus,. We can therefore say that a molecule is the simplest unit of a covalent compound. Atoms combine to form pure elements, compounds, and complex forms like computers and phones. Atomic number, mass number, and isotopes. As such, the atom is the basic building block of chemistry. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is. The nucleus, the center of atom contain proton and neutron, and the outer portion of the atom holds electrons in its orbit around the nucleus [1]. Atoms combine to form pure elements, compounds, and complex forms like computers and phones. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. Web the three. Web 961,030 views • oct 30, 2017 • introduction to anatomy and physiology. Web the atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. The development of modern atomic theory revealed much about the inner structure of atoms. Atoms have protons and neutrons in the. Identify and explain exceptions to predicted electron configurations for atoms and ions. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Web an atom is composed of two regions: Its facts, meaning, size, structure & parts described with examples & picture. Write and interpret symbols that depict the atomic number, mass number,. The development of modern atomic theory revealed much about the inner structure of atoms. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. An atom has a central. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Calculate average atomic mass and isotopic abundance. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web in this article, the structure of an atom, you have understood what an atom is, the parts of a bit, the properties of the fundamental particles of an atom, i.e., protons, electrons, and neutrons. As such, the. Relate electron configurations to element classifications in. The mass of an atom is determined by the total number of protons and neutrons. The structure of the atom. In this tutorial on atomic structure, you will learn about the different parts of the atom, along with the subatomic particles found in each region. Web when atoms combine by forming covalent bonds,. Web structure of the atom. An atom has a central. Its facts, meaning, size, structure & parts described with examples & picture. In this tutorial on atomic structure, you will learn about the different parts of the atom, along with the subatomic particles found in each region. Web when atoms combine by forming covalent bonds, the resulting collection of atoms. A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Basic diagram of an atom most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The structure of the atom. Web to draw the. Web introduction to the atom. Elements are defined by the atomic number, the number of protons in the nucleus. Atoms are the smallest particle of matter than cannot be further subdivided using chemical means. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. Atoms combine to form pure elements, compounds, and complex forms like computers and phones. Download complete chapter notes of structure of atom. Calculate average atomic mass and isotopic abundance. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). Web an atom is composed of two regions: Atomic number, mass number, and isotopes. The negatively charged particles called electrons revolve around the centre of the nucleus. In this tutorial on atomic structure, you will learn about the different parts of the atom, along with the subatomic particles found in each region. If you understand how protons and electrons relate to one another, as well as how neutrons aid in comprising atomic mass, the rest is cake. We can therefore say that a molecule is the simplest unit of a covalent compound. Define the atomic mass unit and average atomic mass. Web rutherford’s gold foil experiment.Diagram Of The Atom
The Structure of the Atom GCSE Physics Science) AQA
Atom Definition, Structure & Parts with Labeled Diagram
Atom Definition, Structure & Parts with Labeled Diagram
Label The Parts Of A Atom
Lets Get Inside An Atom!! The Science Station
Skills Practice AMAZING 8TH GRADE SCIENTISTS
Structure of an Atom Structure & Use of Electron & Proton in Electronics
Label Parts of an Atom — Learning in Hand with Tony Vincent
How to draw Atom structure diagram step by step l Atomic structure
Then Play A Game To Test Your Ideas!
Web 961,030 Views • Oct 30, 2017 • Introduction To Anatomy And Physiology.
An Atom Has A Central.
As Such, The Atom Is The Basic Building Block Of Chemistry.
Related Post: