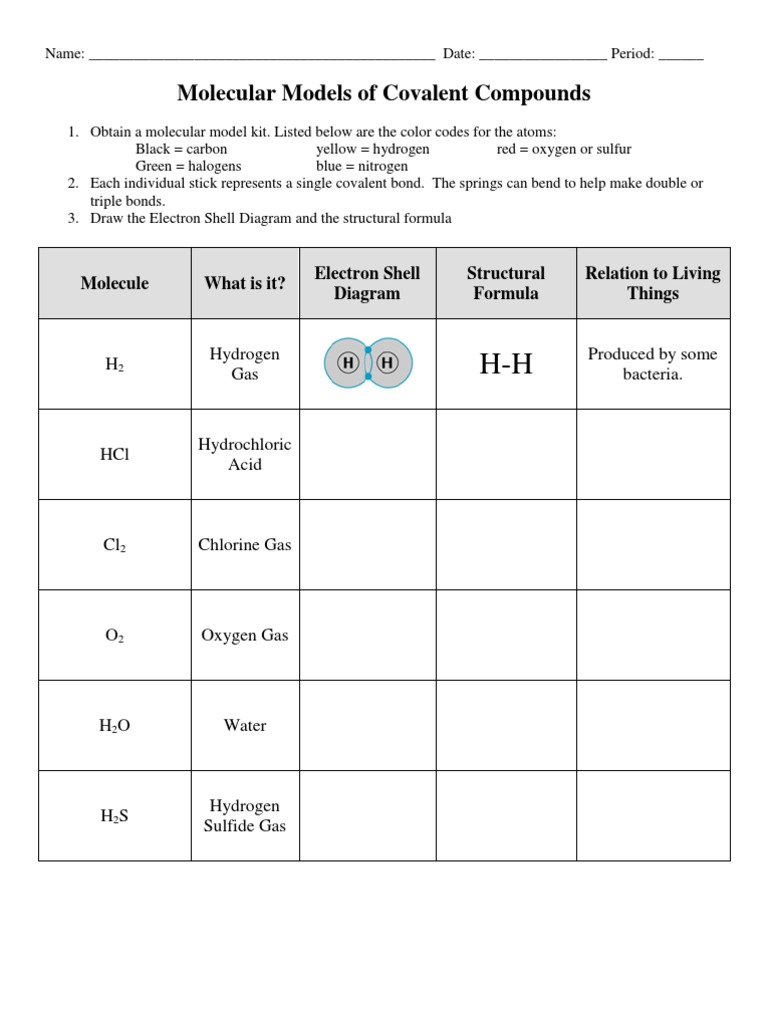
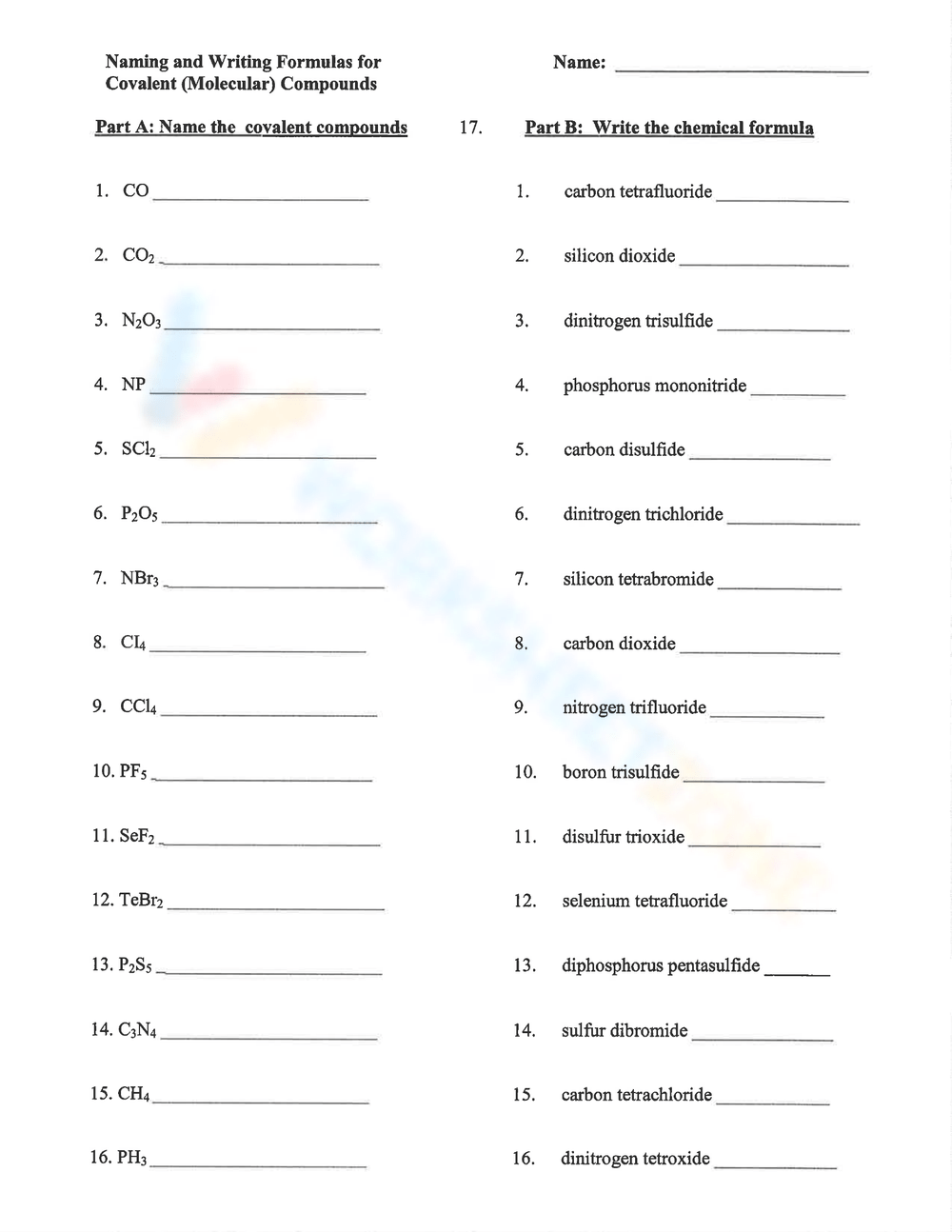
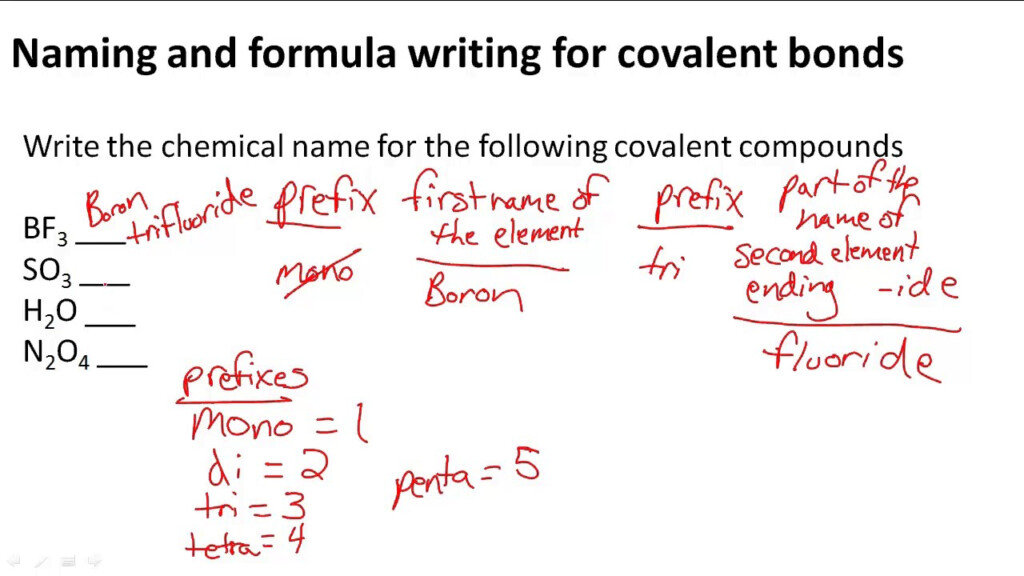
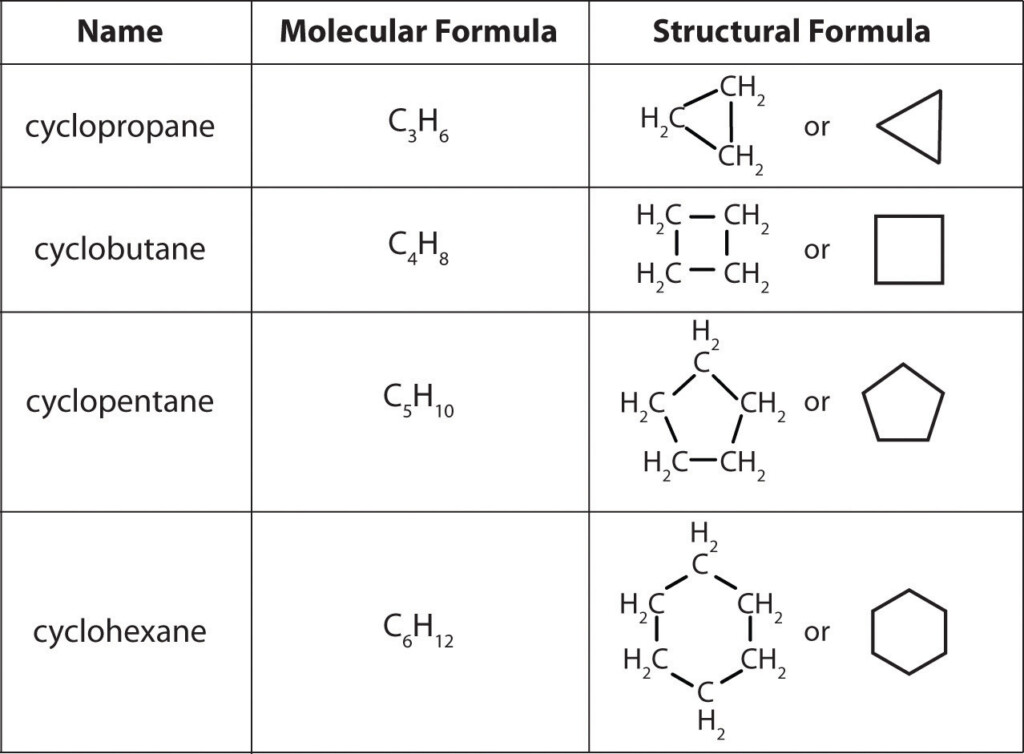
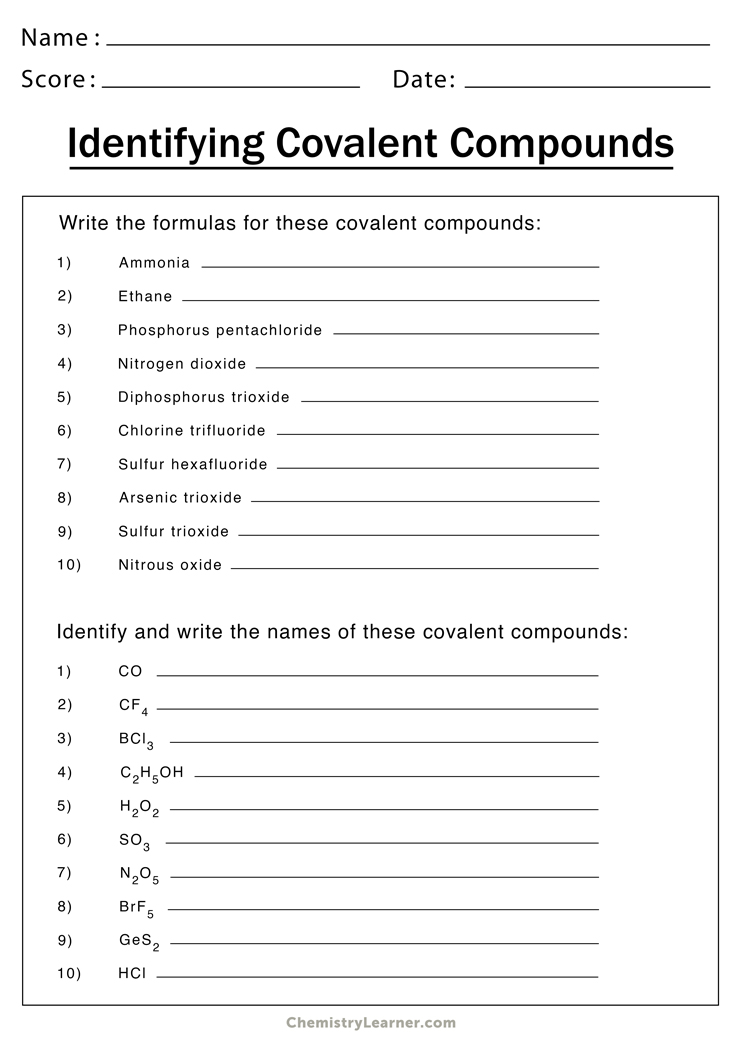
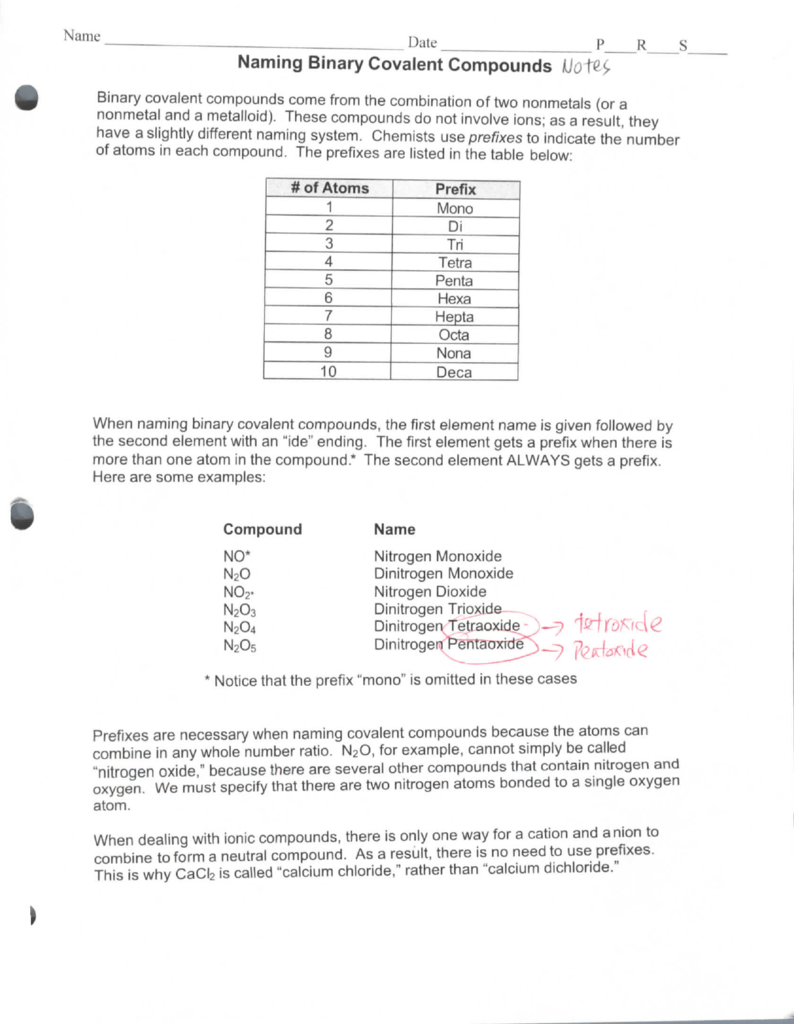
Drawing Covalent Compounds Worksheet
Drawing Covalent Compounds Worksheet - These problems are for practice only will not be graded. Draw the molecular structure of water and label the partial charges of each element. Students are asked to use different colored pencils to show where the electrons came from. Determine the shape of the molecule. This worksheet asks pupils to complete dot and cross diagrams (they only need to add the electrons) and is suitable for a low ability group, or as an introduction/ revision for covalent bonding for middle/higher groups. Molecular compounds can be represented by molecular formulas, which tell how many of each type of atom are in the compound. Web illustrate covalent bond formation with lewis electron dot diagrams. For example, consider ccl4 and nf3 as drawn below: Web this chemistry homework page is perfect for students to practice drawing electron dot diagrams or lewis structures for covalent compounds that have double or triple bonds. Web answer the following questions and check your answers below. Web the atoms in a molecular compound are held together by covalent bonds. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons to fill the first shell. Web this worksheet clearly explains how to draw dot and cross diagrams for covalent compounds, using cl2 as an example. Web illustrate covalent bond formation with lewis electron dot diagrams. Draw a lewis dot diagram for each element listed. Web model1 substances are called ionic compounds and 2 substances are called covalent molecules. Draw the structure of hexane and describe its. Notice that the atoms share electrons so that they all have 8 electrons. Draw the molecular structure of water and label the partial charges of each element. Web covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their outer shell for at least part of the time. Draw the lewis structures for each of the following molecules. Web for the following polyatomic ions, write the ion’s name, calculate the number of total valence electrons (being sure to take the charge into account) and draw the correct lewis structures. Web naming covalent compounds(type iii) worksheet. Web this worksheet clearly explains how to draw dot and cross diagrams for covalent compounds, using cl2 as an example. This worksheet asks pupils to complete dot and cross diagrams (they only need to add the electrons) and is suitable for a low ability group, or as an introduction/ revision for covalent bonding for middle/higher groups. Web drawing lewis structures for covalent compounds. Web this chemistry homework page is perfect for students to practice drawing electron dot diagrams or lewis structures for covalent compounds that have double or triple bonds. For covalent bonding, we often want to draw how the atoms share electrons in the molecule. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Each line represents two electrons. Students are asked to use different colored pencils to show where the electrons came from. (remember, for polyatomic ions, the normal. Web practice drawing lewis dot structures, identifying elements by their structures, and predicting bond and ion formation. Draw the lewis structures for each of the following molecules. Web draw lewis structures for covalent compounds. Place remaining valence electrons to complete the octets of the atoms around the central atom. A lewis or electron dot structure is a convenient representation of the valence electrons in an atom. An electron dot structure for an atom is simply the symbol for the element, surrounded by a number of dots equal to the number of valence electrons. Web. How does the shape of the water molecule determine its polarity? Determine the shape of the molecule. Web naming covalent compounds(type iii) worksheet. Draw the structure of hexane and describe its. Web covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their outer shell for at least part of the. Determine which atoms are bonded together and put two electrons between them to represent the bond 3. Web lewis structures practice worksheet. Each line represents two electrons. Students are asked to use different colored pencils to show where the electrons came from. Pupils are then asked to practise drawing dot and cross diagrams. Web lewis structures practice worksheet. Draw lewis structures depicting the bonding in simple molecules. Notice that the atoms share electrons so that they all have 8 electrons. To instruct the student on how to draw the lewis dot structures for simple, covalent compounds. Determine the shape of the molecule. For covalent bonding, we often want to draw how the atoms share electrons in the molecule. Count the total number of valence electrons for the molecule 2. Did the subscripts (the little numbers shown in the compound formulas) provide any insight into Determine the shape of the molecule. Determine the total number of valence electrons for the formula. Draw lewis structures for the following covalent compounds: The following procedure can be used to draw lewis structure for simple molecules. Draw the lewis structures for each of the following molecules. Draw the structure of hexane and describe its. Be sure you know how to draw correct lewis dot structures and are able to correctly predict the electronic arrangement and. Draw the structure of hexane and describe its. Did the subscripts (the little numbers shown in the compound formulas) provide any insight into A lewis or electron dot structure is a convenient representation of the valence electrons in an atom. Determine the shape of the molecule. This worksheet asks pupils to complete dot and cross diagrams (they only need to. Determine the approximate bond angles. To instruct the student on how to draw the lewis dot structures for simple, covalent compounds. Write a simple rule that will allow you to classify compounds as ionic or covalent on the basis of what you have learned from the model. Place remaining valence electrons to complete the octets of the atoms around the. Place remaining valence electrons to complete the octets of the atoms around the central atom. Why is the water molecule’s shape bent and not linear? Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons to fill the first shell. Lewis structures are representations of molecules that include not only. The following procedure can be used to draw lewis structure for simple molecules. Did the subscripts (the little numbers shown in the compound formulas) provide any insight into Chemistry worksheet lewis dot structures name: Web naming covalent compounds(type iii) worksheet. Molecular compounds can be represented by molecular formulas, which tell how many of each type of atom are in the compound. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons to fill the first shell. Web drawing lewis structures for covalent compounds. Draw a lewis dot diagram for each element listed. Determine the total number of valence electrons for the formula. Draw the lewis structures for each of the following molecules. Web drawing lewis dot structures. (remember, for polyatomic ions, the normal. Draw lewis structures for the following covalent compounds: Be sure you know how to draw correct lewis dot structures and are able to correctly predict the electronic arrangement and molecular geometry before going on to the lab assignment. A lewis or electron dot structure is a convenient representation of the valence electrons in an atom.Molecular Models of Covalent Compounds Worksheet
Chemistry Covalent Bonding Worksheet Answers
Naming And Writing Covalent Compounds And KEY Worksheet
Drawing Covalent Bonds Worksheets Answers
Covalent Compounds Worksheet Formula Writing And Naming Ivuyteq
Naming Covalent Compounds Worksheet 5 1
Drawing Covalent Bonds Worksheet With Answers
Naming covalent compounds worksheet Fill out & sign online DocHub
Naming Covalent Compounds Worksheets Free Printable
Covalent Compounds Worksheet Answer Key Kid Worksheet Printable
Why Is The Water Molecule’s Shape Bent And Not Linear?
This Worksheet Asks Pupils To Complete Dot And Cross Diagrams (They Only Need To Add The Electrons) And Is Suitable For A Low Ability Group, Or As An Introduction/ Revision For Covalent Bonding For Middle/Higher Groups.
Web Draw The Electron Dot Structure.
Determine The Shape Of The Molecule.
Related Post: