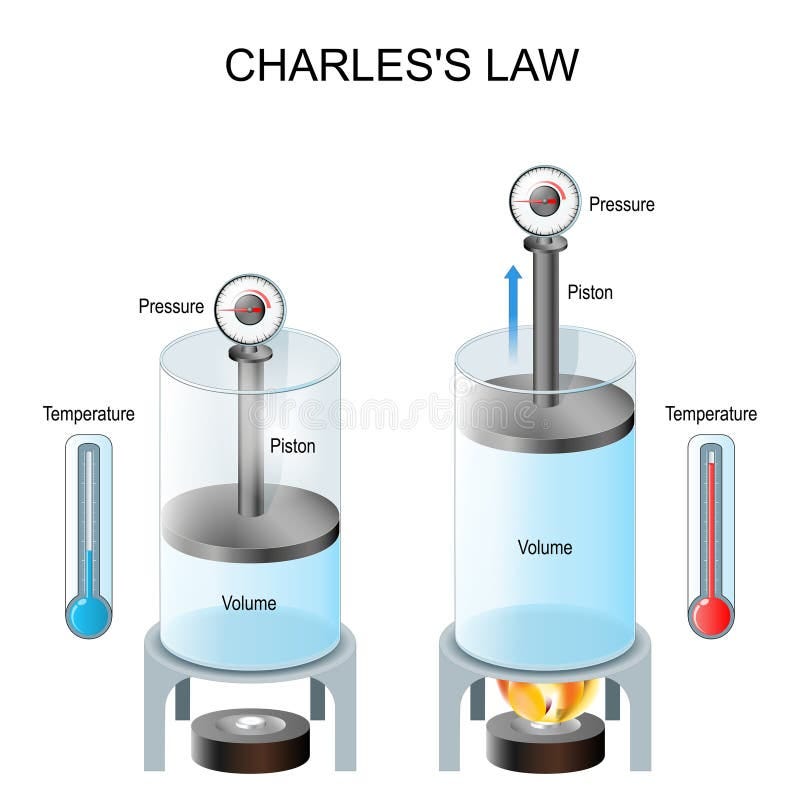
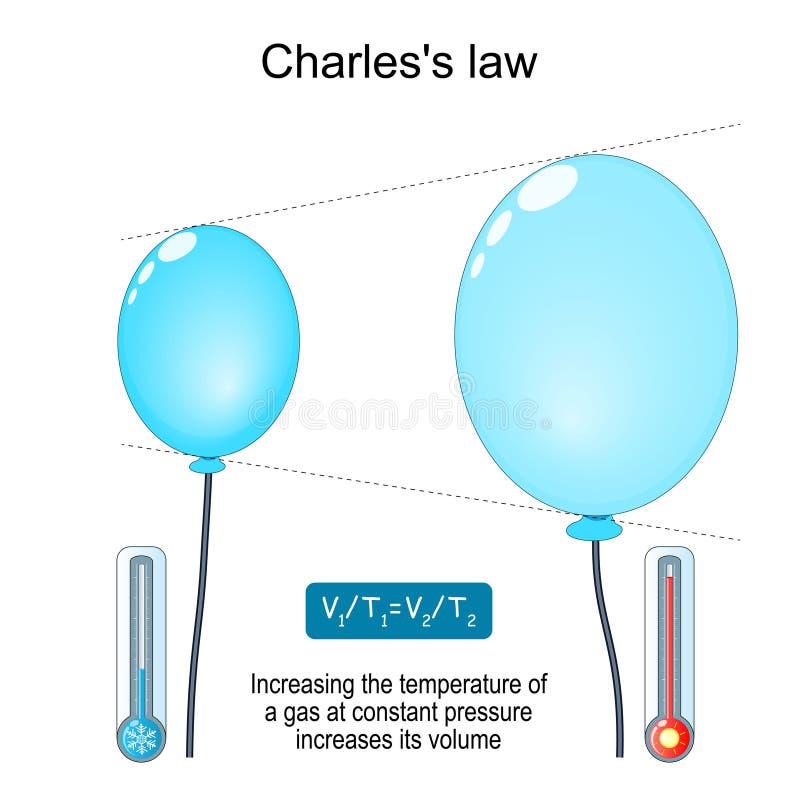
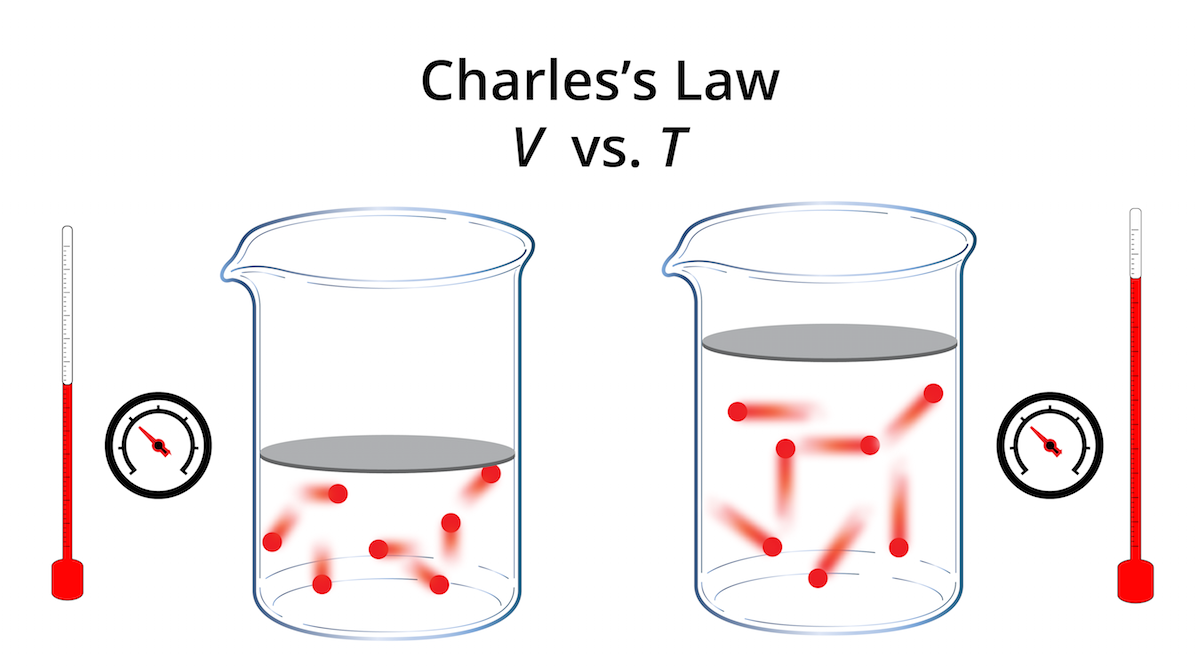
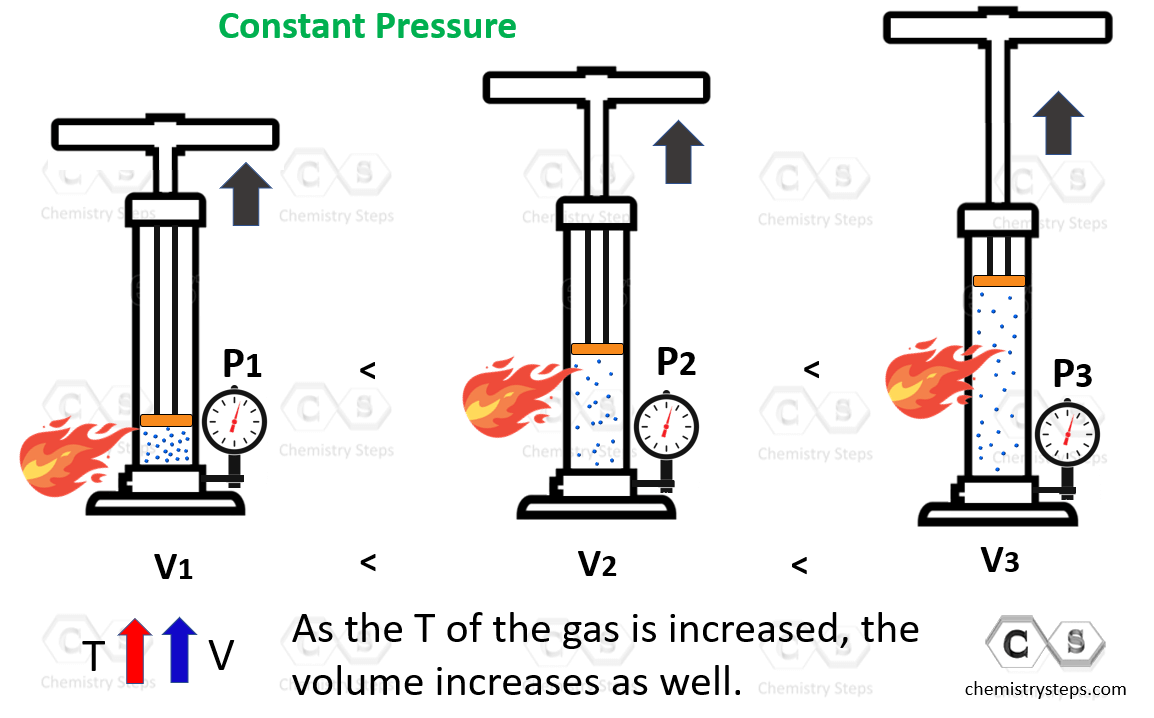
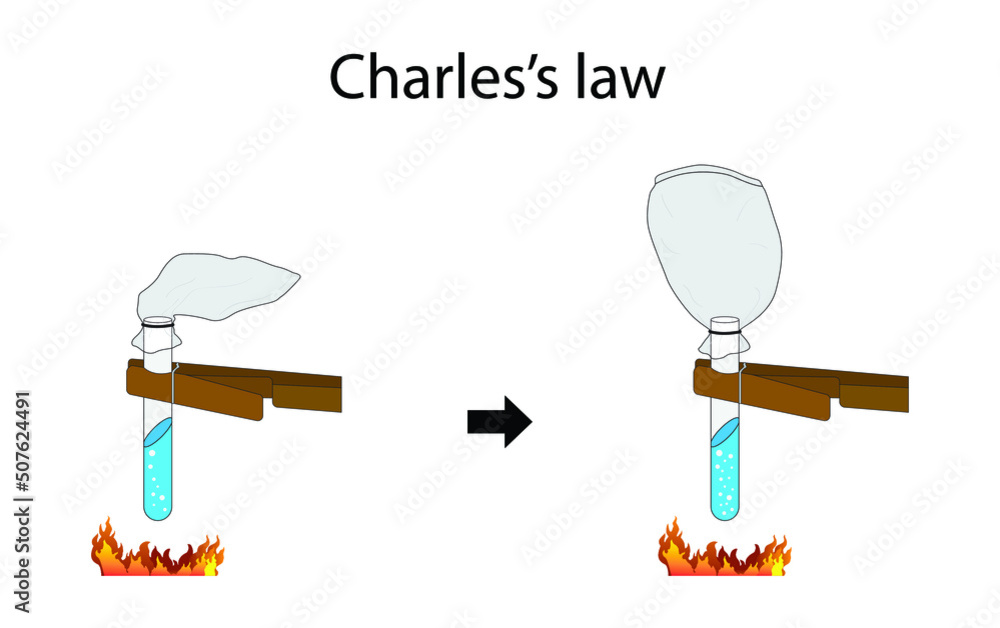
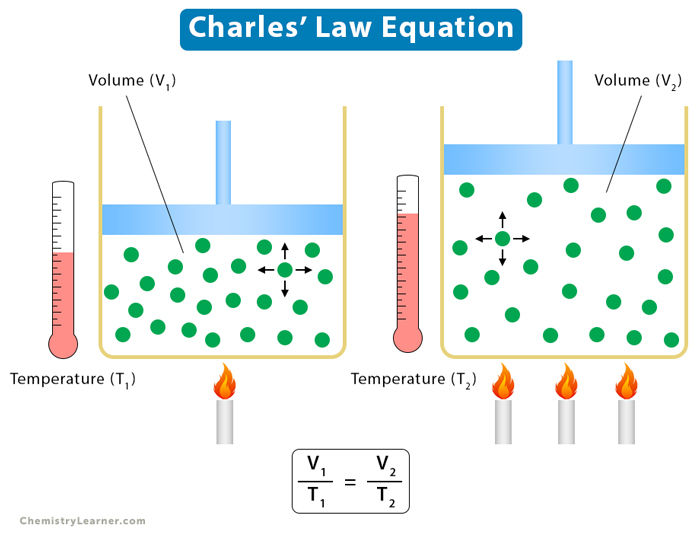
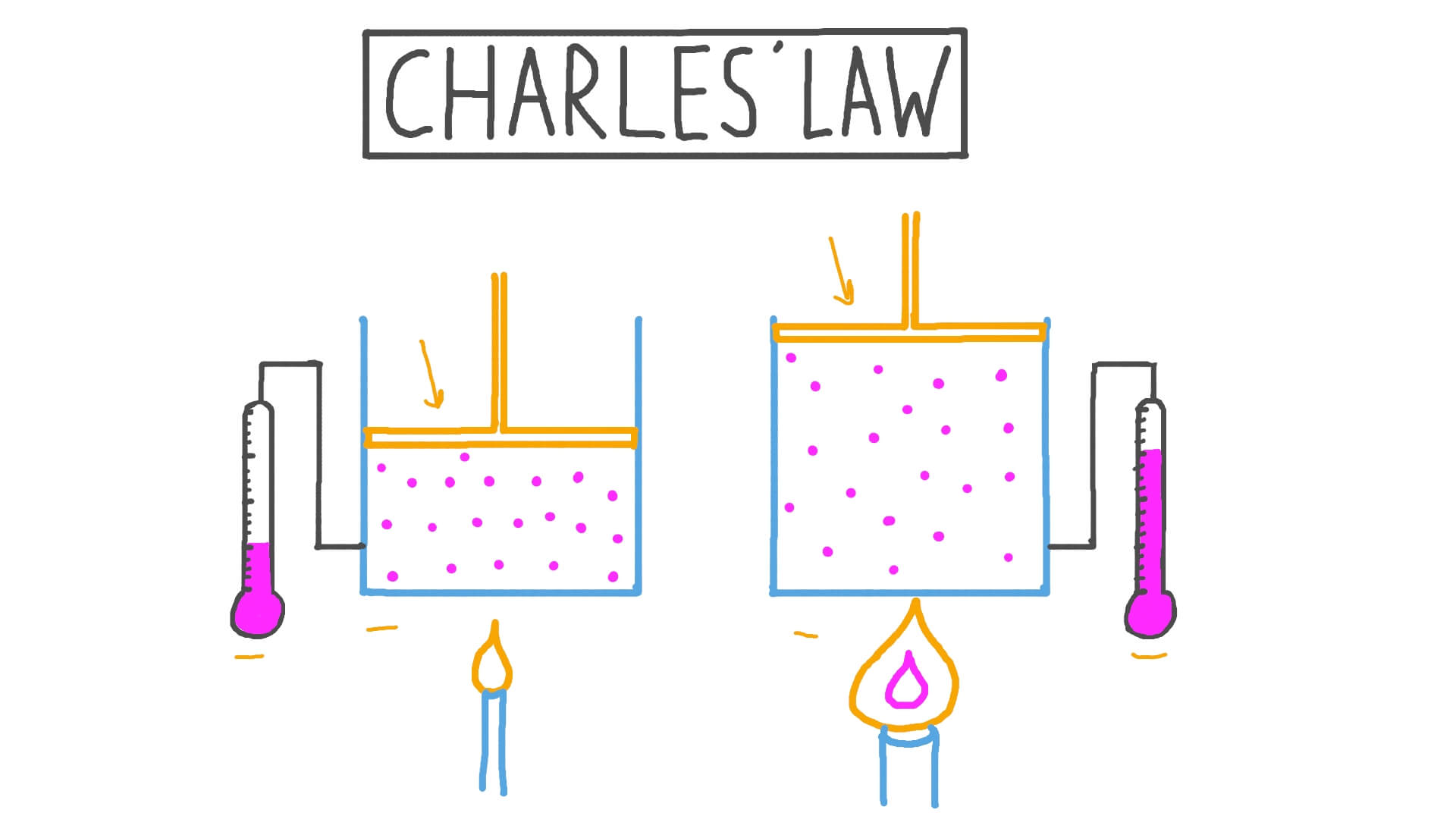
Drawing Of Charles Law
Drawing Of Charles Law - Doubling the temperature of a gas doubles its volume. Boyle's law relates a gas's pressure and volume at constant temperature and amount. In other words, according to charles’s law definition; Web charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. Therefore, v ∝ t or, v = kt, p &. In gas laws, temperatures must always be expressed in kelvins. When we compare the substance under initial ( v₁, t₁) and final conditions ( v₂, t₂ ), you can write charles' law as v₁/t₁ = v₂/t₂. Web charles's law , also known as the law of volumes, explains the relationship between the volume of an ideal gas and its absolute temperature under constant pressure. The volume of a gas increases with an increase in temperature at constant pressure and vice versa. Web charles' law, also known as the law of volumes, describes how gases tend to expand when heated. At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on the absolute temperature scale (i.e. Robert boyle conducted an experiment on gases to study the deviation of its behaviour in changed physical conditions. Let’s assume you get the following data. It can be stated as: Web the behavior of gases can be modeled with gas laws. Web the behavior of gases can be modeled with gas laws. It was first stated by french scientist jacques charles in 1787. The volume (v) of a confined gas (n) is directly proportional to its absolute temperature (t), provided its pressure (p) remains constant. Charles’ law states that the volume occupied by a gas at constant pressure is proportional to its temperature. Web charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. A modern statement of charles' law is: Web the experimental gas law, more commonly known as “charle’s law,” explains the relationship between the volume of a given mass of gas and temperature. At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on the absolute temperature scale (i.e. Web charles’ law can be stated as follows: Charles's law relates a gas's volume and temperature at constant pressure and amount. Web how to demonstrate charles's law. Web charles' law was formulated by the famous scientist jacques charles, less than 2 centuries after boyle’s law, in 1800. In gas laws, temperatures must always be expressed in kelvins. It was first stated by french scientist jacques charles in 1787. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. It states that under a constant temperature when the pressure on a gas increases its volume decreases. Web the experimental gas law, more commonly known as “charle’s law,” explains the relationship between the volume of a given mass of gas and temperature. Web the behavior of gases can be modeled with gas laws. Let’s assume you get the following data.. Web charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. This relationship of direct proportion can be. The pressure is caused by the gas molecules bumping into the walls of the container; Boyle's law relates a gas's pressure and volume at constant temperature. Web charles' law, also known as the law of volumes, describes how gases tend to expand when heated. A modern statement of charles' law is: Web charles' law describes the relationship between the volume and temperature of a fixed mass of gas that is held at a fixed pressure. The absolute temperature is temperature measured with the kelvin scale. This. Boyle's law relates a gas's pressure and volume at constant temperature and amount. So, as the temperature decreases the particles have occupy a smaller volume if the pressure is to remain constant. Charles's law relates a gas's volume and temperature at constant pressure and amount. When we compare the substance under initial ( v₁, t₁) and final conditions ( v₂,. At constant pressure the volume of a given mass of a gas increases or decreases by 1/273 of its volume at 0 o c for every degree rise or fall in temperature. It gives a formal relationship between temperature and volume. So you would do an experiment in which you measure the volume of a gas at various temperatures. The. It can be stated as: Find more details like charles law formula, derivation, and application on this page. When we compare the substance under initial ( v₁, t₁) and final conditions ( v₂, t₂ ), you can write charles' law as v₁/t₁ = v₂/t₂. In gas laws, temperatures must always be expressed in kelvins. At constant pressure, the volume of. Web how to demonstrate charles's law. Web the behavior of gases can be modeled with gas laws. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in kelvins). It can be stated as: It states that under a constant temperature when the pressure on a gas increases its volume decreases. When the pressure on a sample of a dry gas is held constant, the kelvin temperature and the volume will be in direct proportion. The equation for charles's law can be expressed as v 1 /t 1 =v 2 /t 2. Charles's law relates a gas's volume and temperature at constant pressure and amount. Web charles's law , also known. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Therefore, v ∝ t or, v = kt, p &. There’s no duo that takes method dressing more seriously than zendaya and law roach. Charles's law relates a gas's volume and temperature at constant pressure and amount. Web. Web charles' law was formulated by the famous scientist jacques charles, less than 2 centuries after boyle’s law, in 1800. Web how to demonstrate charles's law. Charles's law relates a gas's volume and temperature at constant pressure and amount. In other words, according to charles’s law definition; Web charles law is an ideal gas law that establishes a relation between. The law suggests that the volume of a gas is directly proportional to its temperature when the pressure is kept constant. Web how to demonstrate charles's law. Find more details like charles law formula, derivation, and application on this page. There are four laws, known as gas laws, which describe how gases behave. It can be stated as: Web the behavior of gases can be modeled with gas laws. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. The pressure is caused by the gas molecules bumping into the walls of the container; The absolute temperature is temperature measured with the kelvin scale. Boyle showed that the volume of a sample of a gas is inversely. Also known as the “law of volume,” this law states that volume. Web charles's law states that the volume (v) of a gas is directly proportional to the temperature (t) when pressure is kept constant. This relationship of direct proportion can be. Web charles' law examines the relationship between the volume of a gas and its temperature. Web charles's law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. Web charles’ law is an experimental gas law that describes how gases expand when heated.Chemistry Charles's Law (Gas Laws) with 2 examples Charles law
Charles law volume and temperature Teach Chemistry
Charles's Law Definition, Formula, Examples
Charles S Law. Relationship between Volume and Temperature Stock Vector
Properties of Gases Chemistry Visionlearning
Charle’s Law Chemistry Steps
illustration of physics and chemistry, Charles' law is an experimental
Charless Law Relationship Between Volume Temperature Stock Vector
Charles’ Law Statement, Formula, Examples, and Graph
Unit 1 Behavior of Matter & Properties of Gases
Charles’ Law States That The Volume Occupied By A Gas At Constant Pressure Is Proportional To Its Temperature.
Web Charles's Law , Also Known As The Law Of Volumes, Explains The Relationship Between The Volume Of An Ideal Gas And Its Absolute Temperature Under Constant Pressure.
Web Charles’ Law Can Be Stated As Follows:
V 1 /T 1 = V 2 /T 2.
Related Post: