Helium Atom Drawing
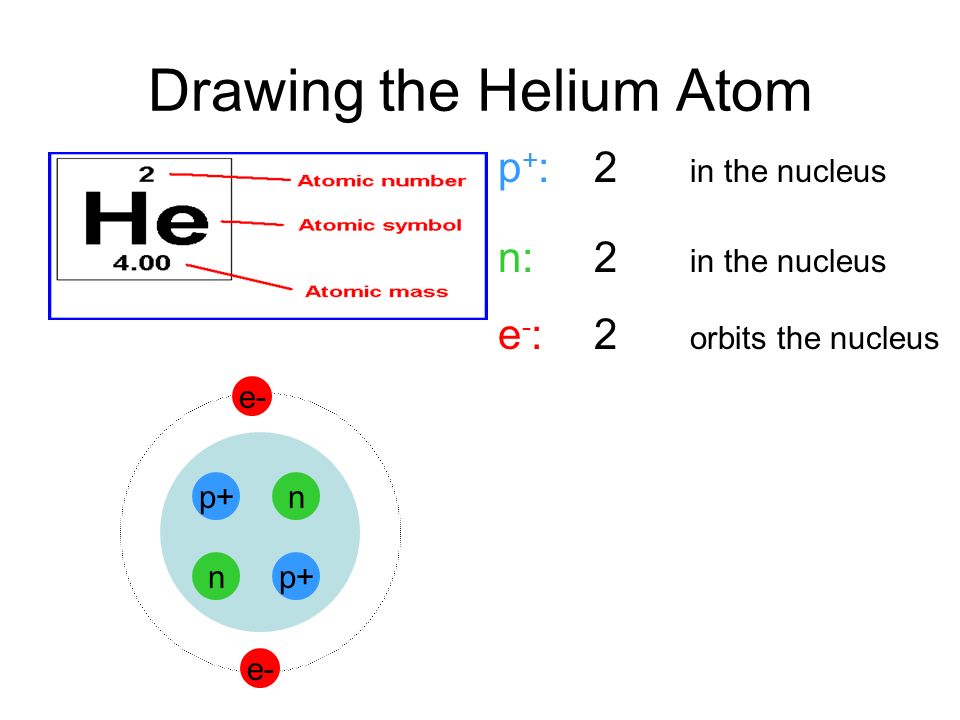
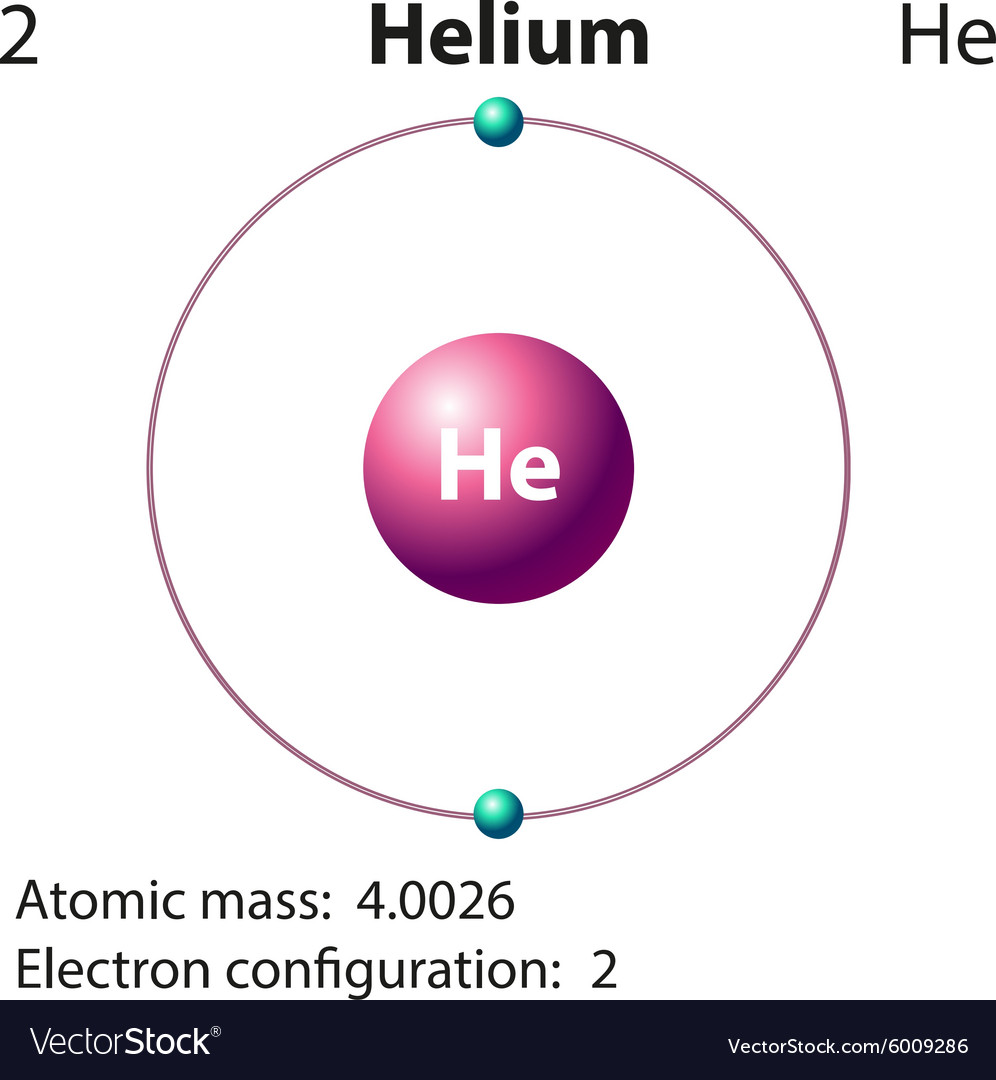
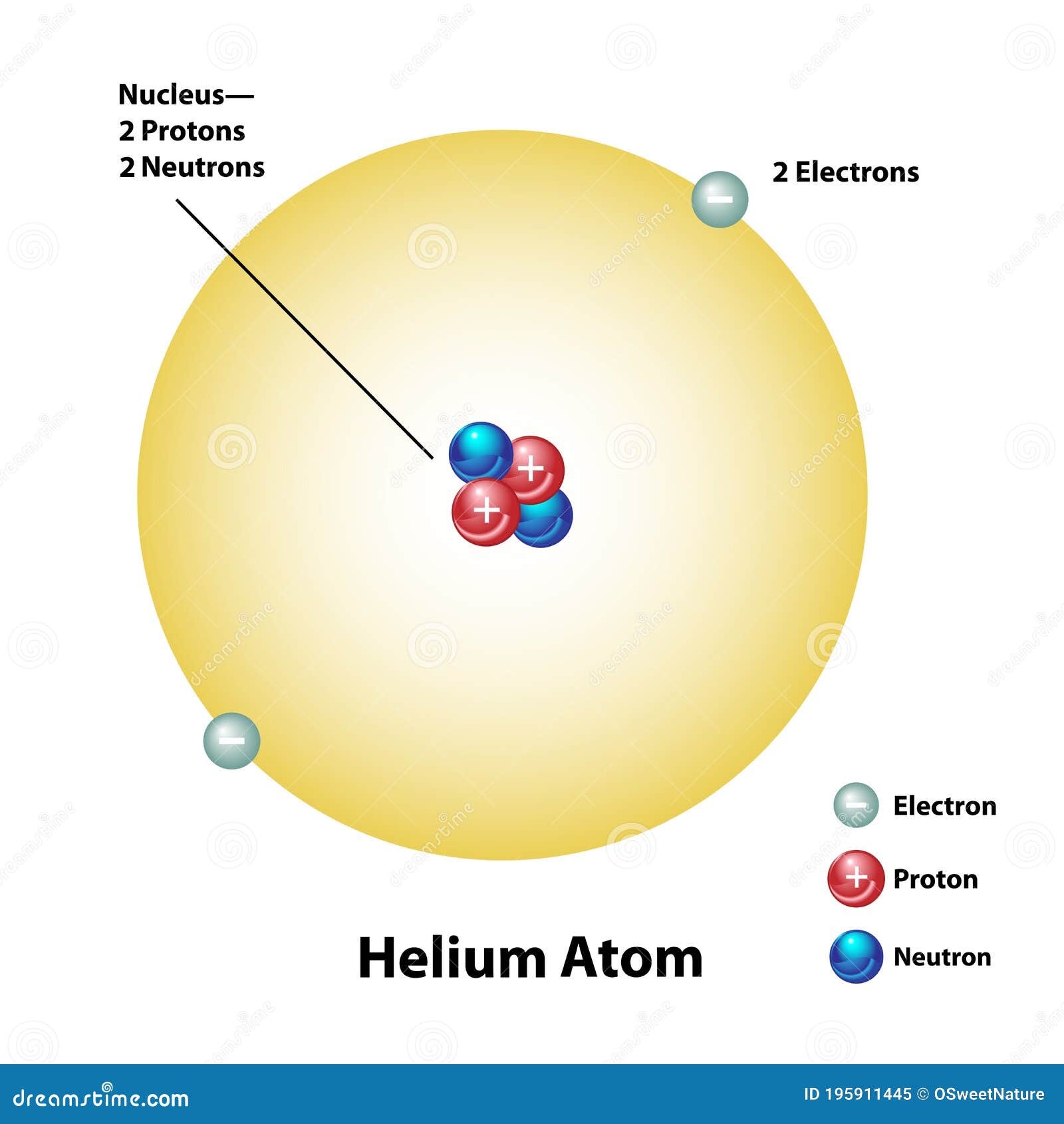
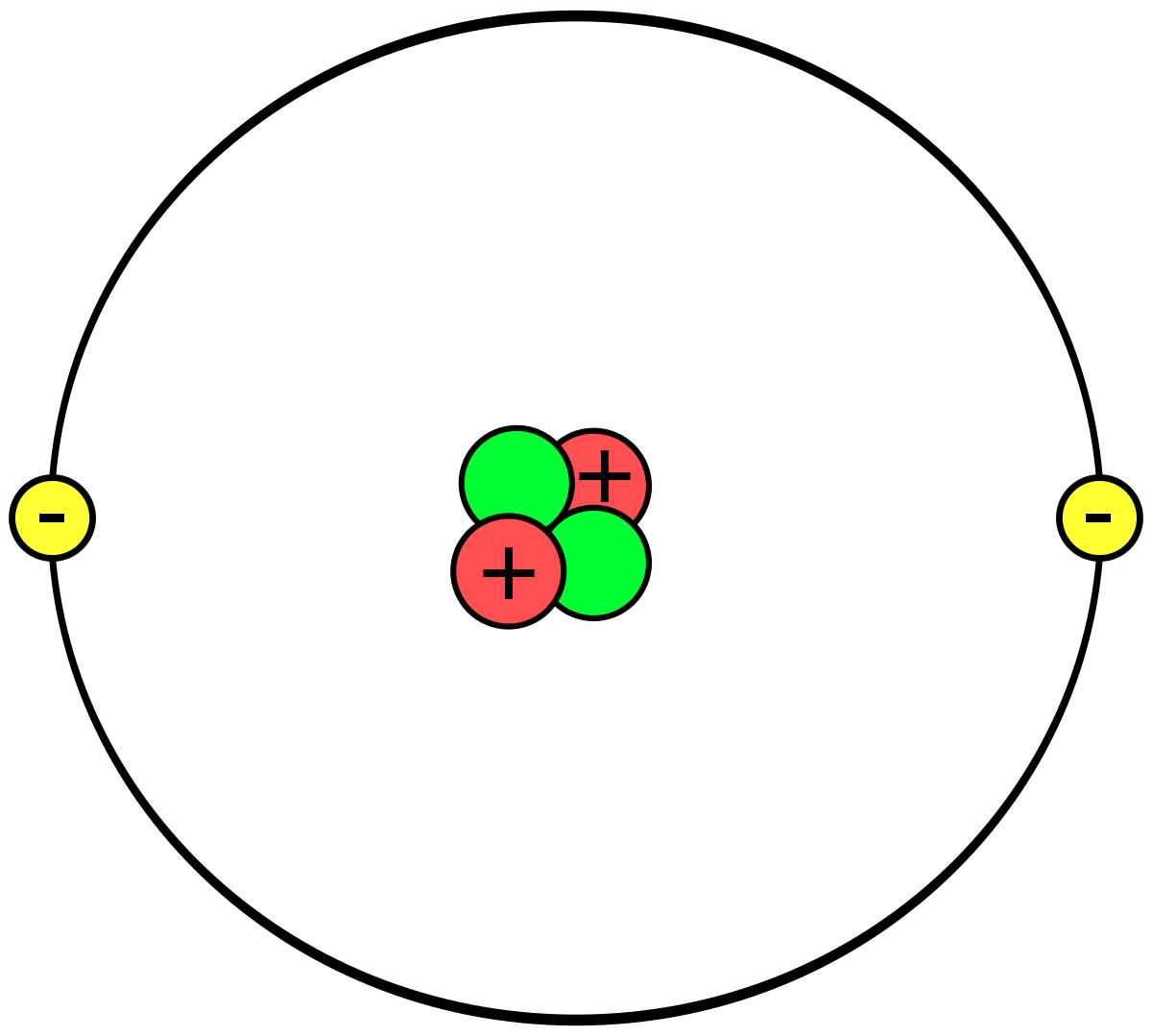
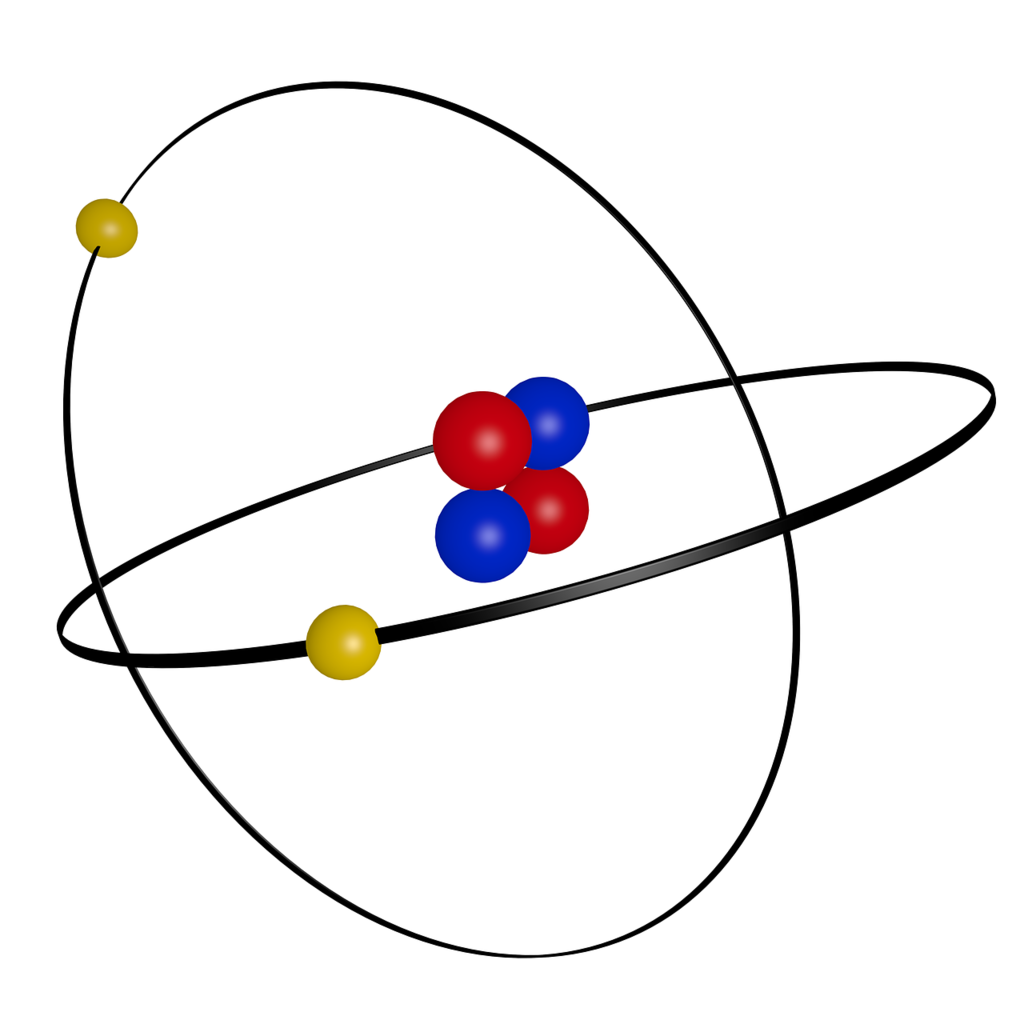
Helium Atom Drawing - We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the he. The first thing we would need to do is to find the total number of valence electrons. Find out why helium is a stable and inert element. Electrons are the negatively charged particles that orbit the nucleus of an atom. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Unlike the other noble gases in group 8, helium only contains two valence electrons. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. In the lewis symbol, the electrons are depicted as two lone pair dots. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Unlike the other noble gases in group 8, helium only contains two valence electrons. Find the number of protons, electrons, and neutrons in the helium atom. It has symbol he and atomic number 2. After discussing the hydrogen atom in the previous lecture, prof. Web a helium atom is an atom of the chemical element helium. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with two neutrons, depending on the isotope , held together by the strong force. Web here we see a schematic diagram of a helium atom in its lowest energy state. Web here's some of the guidelines for drawing dot structures. Web are you looking for the best images of helium atom drawing? To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Unlike the other noble gases in group 8, helium only contains two valence electrons. Web learn how to draw the lewis dot diagram of helium and understand its electron configuration. Helium is one of the noble gases and contains a full valence shell. In the most common variety of helium, the nucleus also contains two neutrons, which have nearly the same mass as the proton but carry no charge. Web here's some of the guidelines for drawing dot structures. 2), the most common isotope of the element helium. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web steps to draw the bohr model of helium atom. I show you where helium is on the periodic table and how to determine how many valence electrons helium has. I show you where helium is on the periodic table and how to determine how many valence electrons helium has. Web here we see a schematic diagram of a helium atom in its lowest energy state. Web here's some of the guidelines for drawing dot structures. 'sun') is a chemical element; 2), the most common isotope of the element helium. Web here we see a schematic diagram of a helium atom in its lowest energy state. Web are you looking for the best images of helium atom drawing? Web the lewis symbol for helium: In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Helium is composed of two electrons bound by the electromagnetic force. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with two neutrons, depending on the isotope , held together by the strong force. In this model, the electrons are represented as black dots that sit on a ring around the nucleus. So let's say we wanted to draw the dot structure. It has symbol he and atomic number 2. Unlike the other noble gases in group 8, helium only contains two valence electrons. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. And we would account for these valence electrons in our dot structure. Electrons are the negatively. The nucleus consists of 2 protons (red) and 2 neutrons (blue). To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. 'sun') is a chemical element; The first thing we would need to do is to find the total number of valence electrons. It has symbol he and atomic number. Web draw a lewis electron dot diagram for an atom or a monatomic ion. The first thing we would need to do is to find the total number of valence electrons. Field explores the next simplest system: The nucleus consists of 2 protons (red) and 2 neutrons (orange). Electrons are the negatively charged particles that orbit the nucleus of an. 2), the most common isotope of the element helium. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. The nucleus is shown as one green circle in the center. Find the number of protons, electrons, and neutrons in the helium atom. Freely sharing knowledge with learners. In the lewis symbol, the electrons are depicted as two lone pair dots. Web in this video we'll look at the atomic structure and bohr model for the helium atom (he). We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the he. Field explores the next simplest system: Protons are the positively charged. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. In the most common variety of helium, the nucleus also contains two neutrons, which have nearly the same mass as the proton but carry no charge. In this model, the electrons are represented as black dots that sit on a ring around the nucleus.. Web in this video we'll look at the atomic structure and bohr model for the helium atom (he). Web are you looking for the best images of helium atom drawing? Field explores the next simplest system: Helium is one of the noble gases and contains a full valence shell. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with two neutrons, depending on the isotope , held together by the strong force. In this model, the electrons are represented as black dots that sit on a ring around the nucleus. The nucleus consists of 2 protons (red) and 2 neutrons (orange). Web the lewis symbol for helium: Electrons are the negatively charged particles that orbit the nucleus of an atom. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. The first thing we would need to do is to find the total number of valence electrons. And we would account for these valence electrons in our dot structure. Freely sharing knowledge with learners.Helium Atom Drawing at Explore collection of
Diagram representation of the element helium Vector Image
Helium Atom with Nucleus and Electron Shell Stock Vector Illustration
Atomic Structure of Helium EthenhasDay
draw atomic structure of helium Brainly.in
Helium Atom Drawing at Explore collection of
Helium Atom Drawing Free download on ClipArtMag
Helium Atom Drawing at GetDrawings Free download
Helium atom Plugon
Web Here's Some Of The Guidelines For Drawing Dot Structures.
Atom Models Represent The Three Main Parts Of An Atom:
The Nucleus Consists Of 2 Protons (Red) And 2 Neutrons (Blue).
We’ll Use A Bohr Diagram To Visually Represent Where The Electrons Are Around The Nucleus Of The He.
Related Post: