Hypertonic Drawing
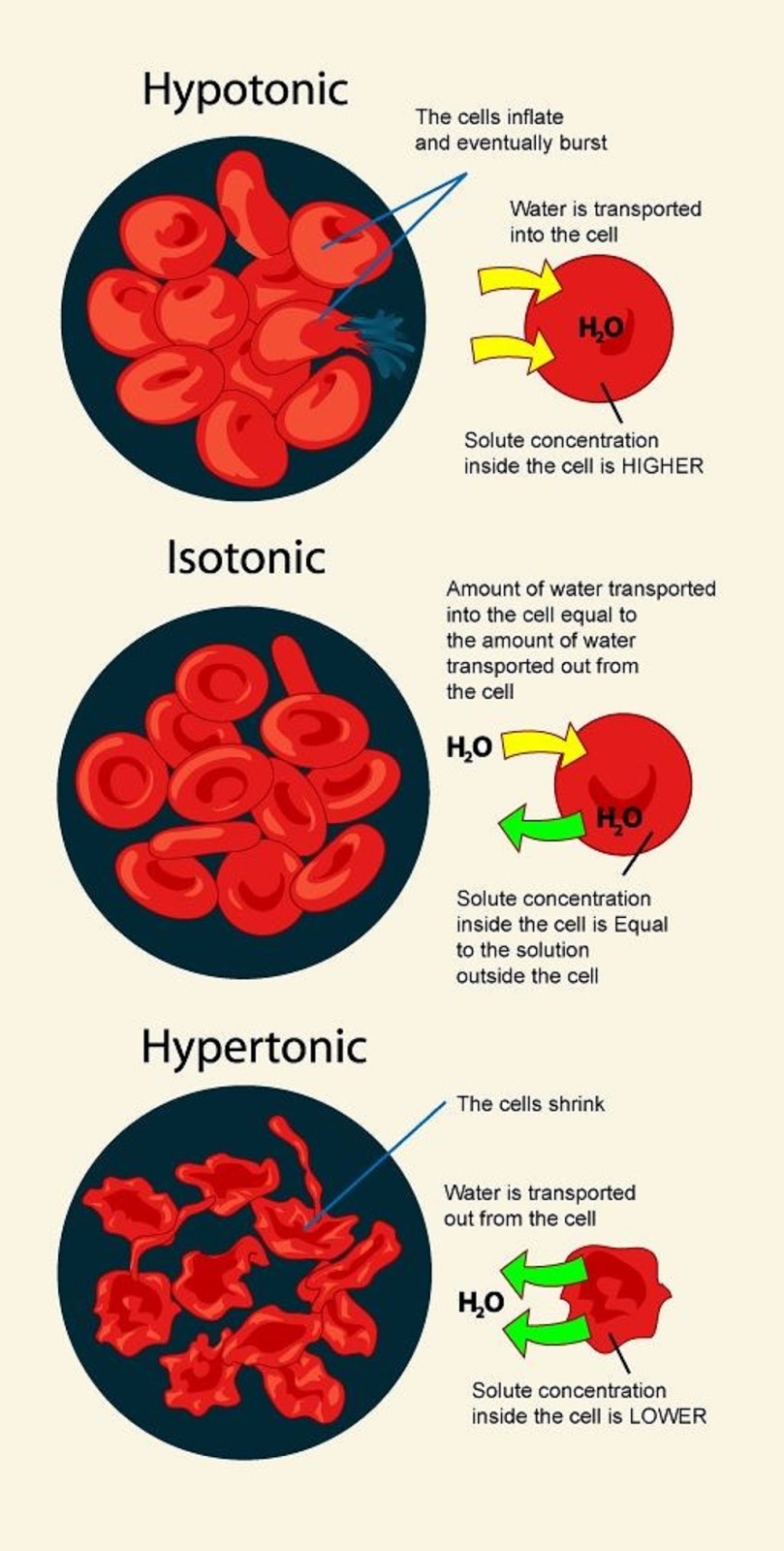
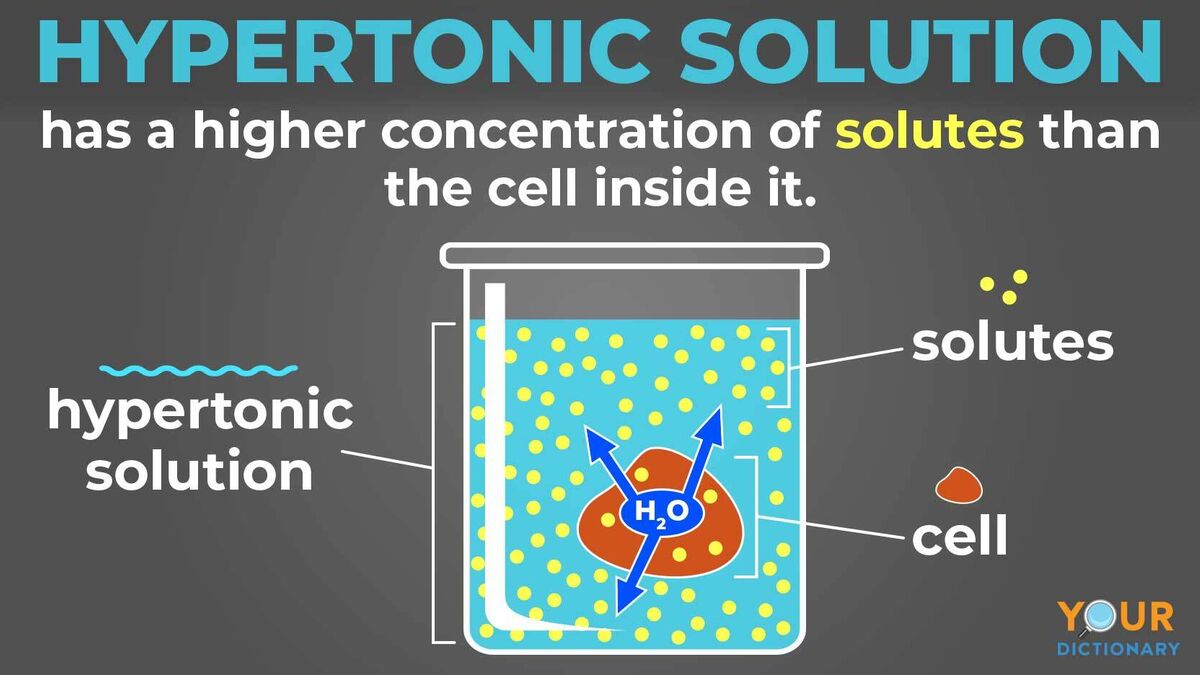
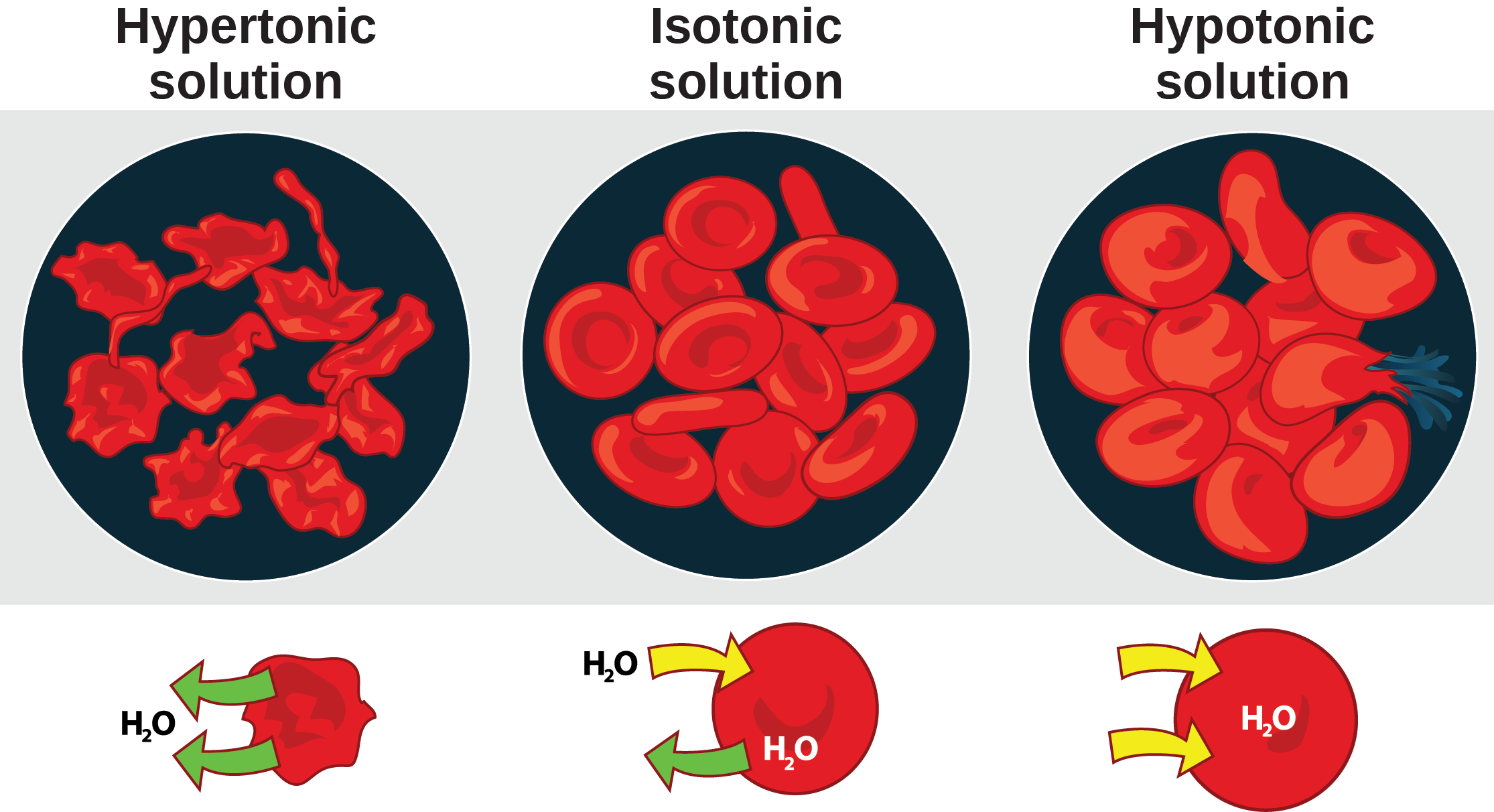
Hypertonic Drawing - Hypertonic, hypotonic and isotonic solutions! What is the difference between hypertonic and hypotonic hydration? Web hypotonic, isotonic and hypertonic solutions (tonicity). Read this study guide to get a deep understanding of these types of solutes. Students will learn about isotonic, hypertonic, and hypotonic solutions and how these solutions affect the movement of water molecules across the cell membrane. In an isotonic solution, there is no net flow of water, keeping the cell stable. Web a hypertonic solution has a higher concentration of solutes compared to another solution across a semipermeable membrane. Web a hypertonic solution contains a higher concentration of solutes compared to another solution. Web what happens when you place an animal cell in a hypertonic solution? Web hypertonic refers to a solution with higher osmotic pressure than another solution. Web to show students how different concentrations in solutions can affect the cell. Web this video is a review of hypotonic, hypertonic and isotonic solutions, how they lead to plasmolysis, cytolysis and dynamic equilibrium. In other words, if there are more solutes outside the cell than inside, water will move out. Web when placed in a hypertonic solution, a red blood cell will lose water and undergo crenation (shrivel). Blood cells suspended in a solution containing more than 0.9% (m/v) sodium chloride solution (saline). Web cells react differently in hypotonic, isotonic, and hypertonic solutions. Web a hypertonic solution has a higher concentration of solutes compared to another solution across a semipermeable membrane. In other words, a hypertonic solution is one in which there is a greater concentration or number of solute particles outside a membrane than there are inside it. If a cell is placed in a hypertonic solution, there will be a net flow of water out of the cell, and the cell will lose volume. Hypotonic solutions, on the other hand, have a lower solute concentration and cause water to move into cells, potentially causing them to swell or burst. Web a hypertonic solution contains a higher concentration of solutes compared to another solution. Read this study guide to get a deep understanding of these types of solutes. Describe the mechanisms of action of hypertonic saline and mannitol. Web a solution having a higher solute concentration or lower water content than another solution is known as a hypertonic solution (latin ‘hyper’ means ‘over’ or ‘above’). 99k views 4 years ago biology. Plant cells in a hypertonic solution can look like a pincushion because of what’s going on inside. Whether a solution is hypertonic or not is measured by comparing the concentration of a solution with another, generally cell sap. Web hypertonic solution the solution whose osmotic pressure is higher than that of the other is called a hypertonic solution. As a result the cell would shrink in what is called plasmolysis. Hypertonic, hypotonic and isotonic solutions! Web this video is a review of hypotonic, hypertonic and isotonic solutions, how they lead to plasmolysis, cytolysis and dynamic equilibrium. Hypotonic hydration swells cells by promoting water intake, whereas hypertonic hydration shrinks them by drawing water out. The opposite solution, with a lower concentration or osmolarity, is known as the hypotonic solution. In a hypotonic solution, water rushes into. Blood cells suspended in a solution containing more than 0.9% (m/v) sodium chloride solution (saline). Web what happens when you place an animal cell in a hypertonic solution? This is clearly seen in red blood cells undergoing a process called crenation. Outline the route of administration and appropriate dosing for hypertonic fluids. A hypotonic solution has a lower concentration of. Hypertonic, hypotonic and isotonic solutions! Outline the route of administration and appropriate dosing for hypertonic fluids. In an isotonic solution, there is no net flow of water, keeping the cell stable. This causes water to rush out making the cell wrinkle or shrivel. Students will learn about isotonic, hypertonic, and hypotonic solutions and how these solutions affect the movement of. Web three terms—hypertonic, hypotonic, and isotonic—are used to describe whether a solution will cause water to move into or out of a cell: Web hypertonic solutions can make it easier for the kidneys to remove excess water from your patient’s body. Web in this video we discuss the three types of osmotic solutions: Web a hypertonic solution has a higher. Describe the mechanisms of action of hypertonic saline and mannitol. Seeing the effect of various types of solution on the direction of osmosis. If a cell is placed in a hypertonic solution, there will be a net flow of water out of the cell, and the cell will lose volume. Blood cells suspended in a solution containing more than 0.9%. This is clearly seen in red blood cells undergoing a process called crenation. Plant cells in a hypertonic solution can look like a pincushion because of what’s going on inside. What is the difference between hypertonic and hypotonic hydration? Web three terms—hypertonic, hypotonic, and isotonic—are used to describe whether a solution will cause water to move into or out of. Web when placed in a hypertonic solution, a red blood cell will lose water and undergo crenation (shrivel). In this case, water will leave the cell since the cell has a lower osmolarity than the extracellular fluid. What is the difference between hypertonic and hypotonic hydration? Describe the mechanisms of action of hypertonic saline and mannitol. Web hypertonic solution the. Hypotonic hydration swells cells by promoting water intake, whereas hypertonic hydration shrinks them by drawing water out. If a cell is placed in a hypertonic solution, there will be a net flow of water out of the cell, and the cell will lose volume. Hypertonic, hypotonic and isotonic solutions! Web hypertonic solutions have a higher solute concentration and cause water. Review the adverse effects and contraindications of hypertonic saline and mannitol. Web cells react differently in hypotonic, isotonic, and hypertonic solutions. In a hypotonic solution, water rushes into the cell causing it to expand or even burst. Web hypertonic solutions can make it easier for the kidneys to remove excess water from your patient’s body. A hypotonic solution has a. Whether a solution is hypertonic or not is measured by comparing the concentration of a solution with another, generally cell sap. In an isotonic solution, there is no net flow of water, keeping the cell stable. In other words, a hypertonic solution is one in which there is a greater concentration or number of solute particles outside a membrane than. This is clearly seen in red blood cells undergoing a process called crenation. In a hypotonic solution, water rushes into the cell causing it to expand or even burst. Hypertonic, hypotonic and isotonic solutions! Web need help in understanding hypotonic vs hypertonic, and isotonic solutions? Whether a solution is hypertonic or not is measured by comparing the concentration of a solution with another, generally cell sap. What is the difference between hypertonic and hypotonic hydration? Web a hypertonic solution has a higher concentration of solute than another solution, meaning water will flow into it. Web a hypertonic solution contains a higher concentration of solutes compared to another solution. The opposite solution, with a lower concentration or osmolarity, is known as the hypotonic solution. Outline the route of administration and appropriate dosing for hypertonic fluids. This causes water to rush out making the cell wrinkle or shrivel. Web in this video we discuss the three types of osmotic solutions: Hypertonic, hypotonic and isotonic solutions! Web a hypertonic solution has a higher concentration of solutes compared to another solution across a semipermeable membrane. In this case, water will leave the cell since the cell has a lower osmolarity than the extracellular fluid. Hypotonic hydration swells cells by promoting water intake, whereas hypertonic hydration shrinks them by drawing water out.effets de hypertonique, hypotonique et istonique solutions à rouge du
Animal Cell In Hypertonic Solution
Medical and Health Science Hypotonic, Isotonic, Hypertonic Solution
Hypertonic Diagram
Osmoregulation and Osmotic Balance OpenStax Biology 2e
Hypertonic, Hypotonic, & Isotonic Diagram Quizlet
Iso, Hypo, and Hypertonic Solutions Diagram Quizlet
Types of solutionshypertonic hypotonic and isotonic explained YouTube
Illustration showing the effect of hypotonic, isotonic and hypertonic
Hypertonic Hypotonic And Isotonic Cells
Web Hypotonic, Isotonic And Hypertonic Solutions (Tonicity).
99K Views 4 Years Ago Biology.
Web To Show Students How Different Concentrations In Solutions Can Affect The Cell.
Web Hypertonic Solution The Solution Whose Osmotic Pressure Is Higher Than That Of The Other Is Called A Hypertonic Solution.
Related Post: