Lewis Structure Drawing Practice
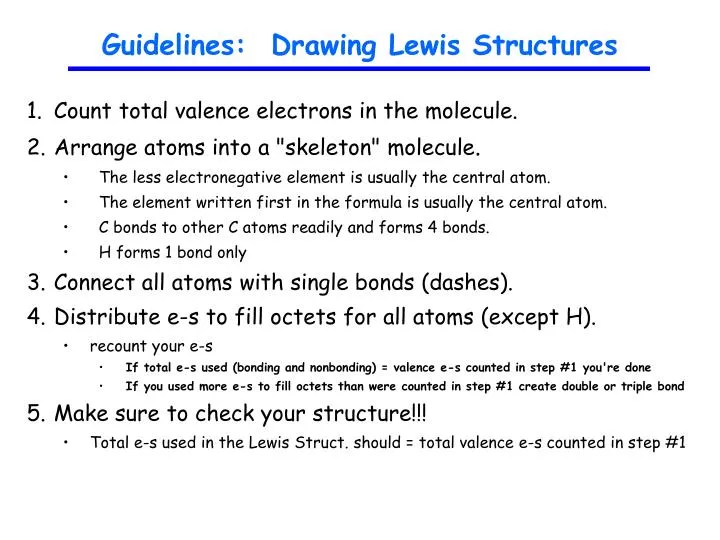
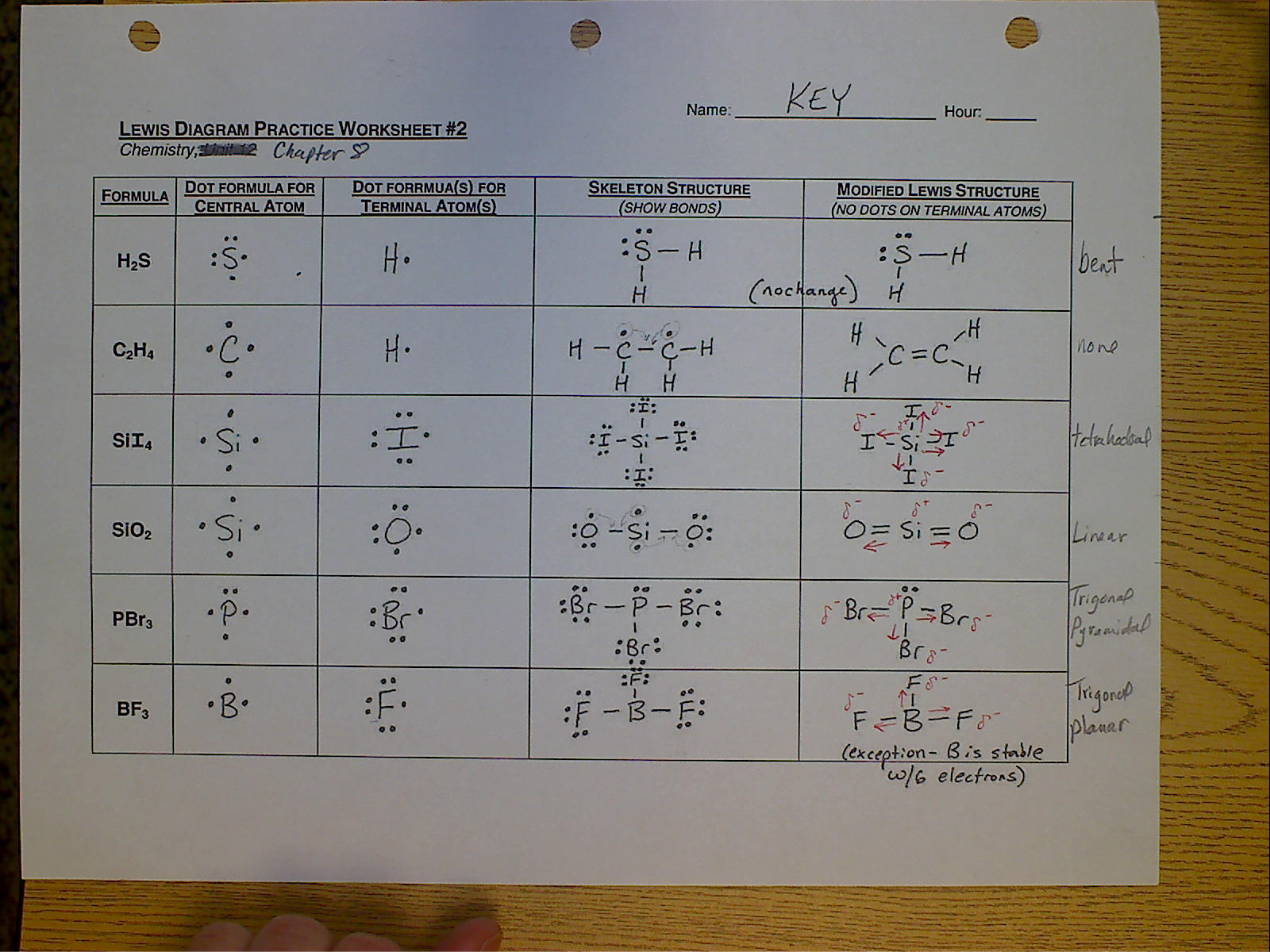
Lewis Structure Drawing Practice - Web draw the lewis structures for ch 3 s(=o)h and ch 3 c(=o)h with the appropriate hybridizations, and explain these observations. Learn the basics of chemical bonding and molecular geometry. Such molecules are very common, and they provide a foundation for understanding structures of more complex molecules. Web in chem 101a, we will focus on drawing lewis structures of molecules and polyatomic ions that have one central atom with several other atoms attached to it. Web lewis structures are drawn by a series of dots, lines, and atomic symbols and provide a structure for the way that the atom or molecule is arranged. Web practise drawing the lewis structure of molecules using the exercises below. See why lewis structures are important. This is the lewis structure we are attempting to create. Web lewis structures and the octet rule. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Determine if the molecule is polar or nonpolar. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web the compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web learn how to draw a lewis structure to show the bonding and valence electrons in a molecule. Ethanethiol, c a 2 h a 6 s , is a clear liquid with a strong odor. Web draw three possible lewis structures for n 2o and assign formal charges to the atoms in each molecule. Learn the basics of chemical bonding and molecular geometry. In short, remember these steps for determining the lewis structure: Web things to remember 1. Write the correct skeletal structure for the molecule. Web practise drawing the lewis structure of molecules using the exercises below. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Web practice drawing lewis dot structures, identifying elements by their structures, and predicting bond and ion formation. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web in these practice problems, we will work on determining the lewis structures of molecules and ions. Web draw lewis structures online with this interactive tool. Carbon (c) will always be the central atom and hydrogen (h) will never be the central atom 2. Web understand what a lewis dot structure is and how to draw it, and practice drawing hydrogen, carbon, and other lewis dot structures. Then name their electron arrangement, shape, and bond angles. With a bit of practice, you can easily create lewis structures for almost any molecule. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Web things to remember 1. Web practice drawing lewis dot structures, identifying elements by their structures, and predicting bond and ion formation. Ethanethiol, c a 2 h a. Web learn how to draw a lewis structure to show the bonding and valence electrons in a molecule. Web practice drawing lewis dot structures, identifying elements by their structures, and predicting bond and ion formation. Web understand what a lewis dot structure is and how to draw it, and practice drawing hydrogen, carbon, and other lewis dot structures. The skeletal. The skeletal structure of ethanethiol is shown below. Web draw lewis structures online with this interactive tool. Web practise drawing the lewis structure of molecules using the exercises below. Ethanethiol, c a 2 h a 6 s , is a clear liquid with a strong odor. Web things to remember 1. Web in these practice problems, we will work on determining the lewis structures of molecules and ions. Web practise drawing the lewis structure of molecules using the exercises below. These problems are for practice only will not be graded. (diacetylene may be a little tricky!) answer. Web lewis structures and the octet rule. If you are not sure if your structure is correct, do a formal charge check. Web many planets in our solar system contain organic chemicals including methane (ch 4) and traces of ethylene (c 2 h 4 ), ethane (c 2 h 6 ), propyne (h 3 ccch), and diacetylene (hcccch). So let's say we wanted to draw the dot. Learn the basics of chemical bonding and molecular geometry. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Then, identify the most stable structure for n 2 o. Carbon (c) will always be the central atom and hydrogen (h) will never be the central atom 2. Write the correct skeletal structure for the. If you are not sure if your structure is correct, do a formal charge check. This is the lewis structure we are attempting to create. Web the compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. In other cases choose the element that is the least electronegative (farthest to the left). Learn the basics of chemical bonding and molecular geometry. A lewis dot structure can be made for a single atom, a covalent compound, or a polyatomic ion. The compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. In short, remember these steps for determining the lewis structure: Web practise drawing the. The skeletal structure of ethanethiol is shown below. Web drawing lewis structures guide. Web practice drawing lewis dot structures, identifying elements by their structures, and predicting bond and ion formation. In short, remember these steps for determining the lewis structure: * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Let's consider nitromethane shown below. Web answer the following questions and check your answers below. Web practice drawing lewis dot structures, identifying elements by their structures, and predicting bond and ion formation. Web drawing lewis structures guide. Web draw the lewis structures for ch 3 s(=o)h and ch 3 c(=o)h with the appropriate hybridizations, and explain these observations. This is the lewis structure we are attempting to create. Web practice drawing lewis structures with answers and explanation. Web draw the lewis structures for ch 3 s(=o)h and ch 3 c(=o)h with the appropriate hybridizations, and explain these observations. Let's consider nitromethane shown below. Web draw the lewis structure for the following molecules. Determine if the molecule is polar or nonpolar. (diacetylene may be a little tricky!) answer. Web draw three possible lewis structures for n 2o and assign formal charges to the atoms in each molecule. In short, remember these steps for determining the lewis structure: In other cases choose the element that is the least electronegative (farthest to the left) on the periodic table. The first thing we would need to do is to find the total number of valence electrons. Write the correct skeletal structure for the molecule. Write the lewis structures for each of these molecules. The compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. The skeletal structure of ethanethiol is shown below. Such molecules are very common, and they provide a foundation for understanding structures of more complex molecules.Beginner's Guide to Drawing Lewis Structures YouTube
How To Draw Lewis Structures A Step By Step Tutorial
How to Draw a Lewis Structure
3 Ways to Draw Lewis Dot Structures wikiHow
How To Draw Lewis Structures A Step By Step Tutorial
Draw Lewis Structure Practice
How to draw Lewis Structures a step by step tutorial Middle School
rules for drawing lewis dot structures for molecules
Draw Lewis Structure Practice
Practice Drawing Lewis Structures Worksheets Worksheets For Kindergarten
Web Drawing Lewis Structures Guide.
Learn The Basics Of Chemical Bonding And Molecular Geometry.
Sum The Valence Electrons From All The Atoms.
Web In These Practice Problems, We Will Work On Determining The Lewis Structures Of Molecules And Ions.
Related Post: