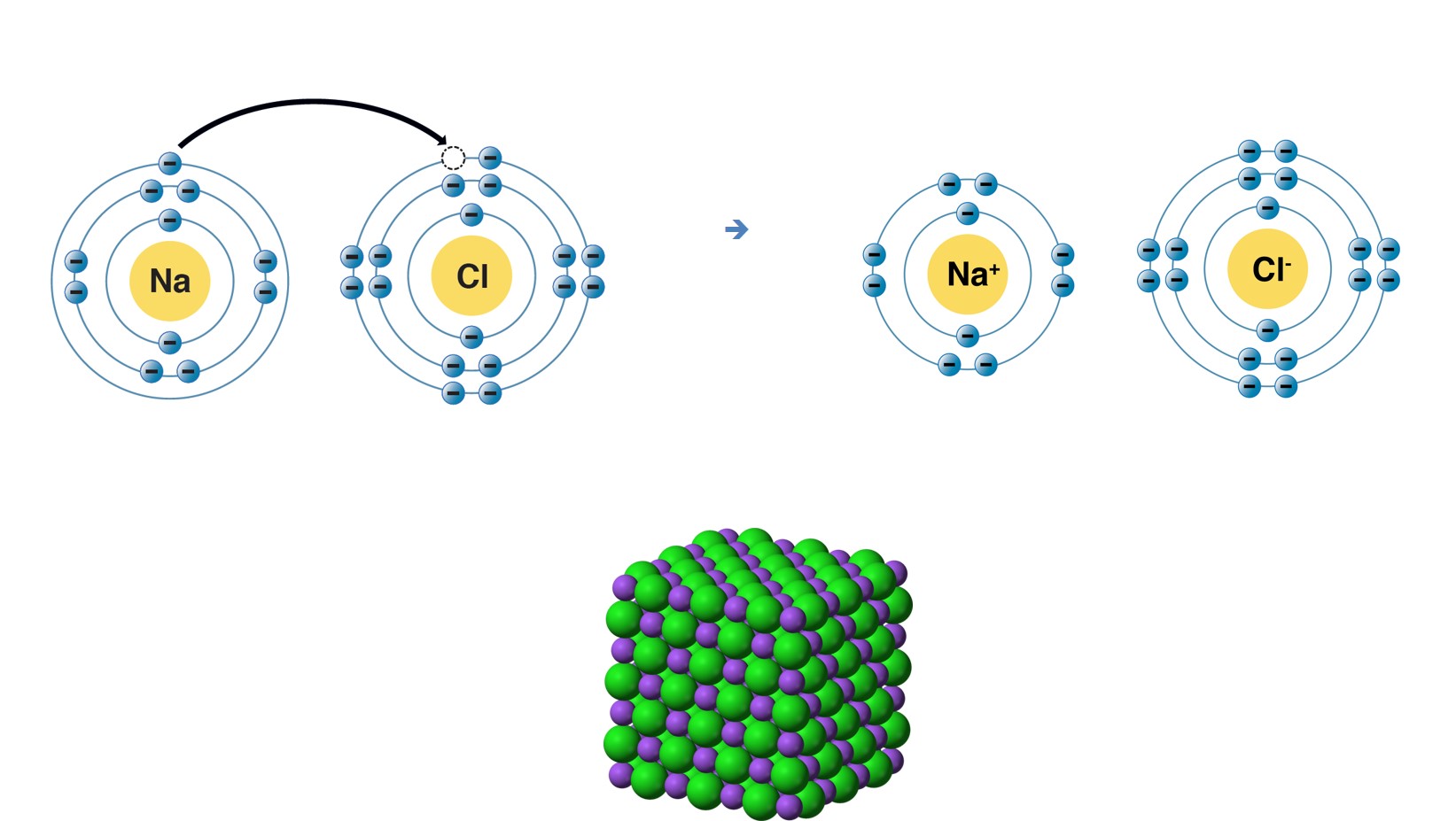
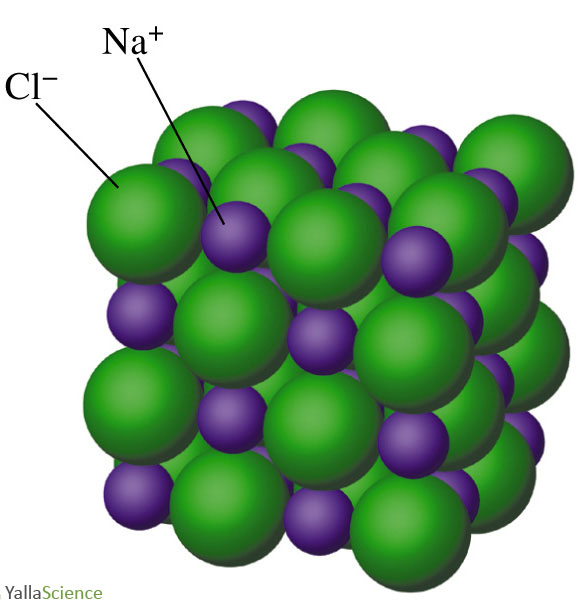
Nacl Drawing
Nacl Drawing - Since they’re from opposite sides of the periodic table, they form an ionic compound. Together, they form nacl, one unit of sodium chloride. The following figure shows how these ions are arranged in. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. Web how to draw the lewis structure of nacl (sodium chloride, ionic) chemistnate. Nacl 6 and clna 6 octahedra. Web sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. Together, they combine to form a unit of nacl. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. We are also attempting to establish a structure with the smallest formal charge possible. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. The following figure shows how these ions are arranged in. Schematic representation of valence electrons of a molecule in line (bonds) and dot (electrons) forms is lewis structure. Since magnesium is in group ii of the periodic table, it forms ions with charge of +2. Web sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. Web lewis dot structure for sodium chloride. This nonstop attack continuous until the whole nacl crystal disintegrates. The structure of nacl is formed by repeating the face centered cubic unit cell. Web structure of sodium chloride. It is extracted from the mineral form halite or evaporation of seawater. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. Web lewis dot structure for sodium chloride. Nacl 6 and clna 6 octahedra. This nonstop attack continuous until the whole nacl crystal disintegrates. Web nacl lewis structure & characteristics: Web how to draw lewis structure of nacl. Since they’re from opposite sides of the periodic table, they form an ionic compound. The structure of sodium chloride. The intramolecular bonding is ionic, as it involves the transfer of electrons from sodium to chlorine, and bonds ions through electrostatic forces. The following figure shows how these ions are arranged in. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. Web how to draw lewis structure of nacl. The structure of nacl is formed by repeating the face centered cubic unit cell. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. Web how to draw lewis structure of nacl. It is extracted from the mineral form halite or evaporation of seawater. Since they’re from opposite sides of the periodic table, they form an ionic compound. Both ions show octahedral coordination (cn = 6). 101k views 4 years ago lewis. 101k views 4 years ago lewis. Web nacl has a cubic unit cell. Ionic crystals depend upon the structure and the size of their ions. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. For the nacl lewis structure, calculate the total number of valence electrons for the nacl m.more. Together, they form nacl, one unit of sodium chloride. Web crystal structure of sodium chloride. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g. The structure of nacl is formed by repeating the face centered cubic unit cell. Web crystal structure of sodium chloride. The cell looks the same whether you start with anions or cations on the corners. Web nacl lewis structure & characteristics: It is extracted from the mineral form halite or evaporation of seawater. It is extracted from the mineral form halite or evaporation of seawater. Web lewis dot structure for sodium chloride. Schematic representation of valence electrons of a molecule in line (bonds) and dot (electrons) forms is lewis structure. When drawing a lewis dot structure, we are always attempting to achieve an electron count at which all of the atoms involved are stable and (usually) have full octets. 101k views 4 years ago lewis.. The cell looks the same whether you start with anions or cations on the corners. Web structure of sodium chloride. The structure of nacl is formed by repeating the face centered cubic unit cell. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. Ionic crystals depend upon the structure and the size of their ions. The structure of nacl is formed by repeating the face centered cubic unit cell. We are also attempting to establish a structure with the smallest formal charge possible. In this model, it is a representation of the structure of sodium chloride, an ionic network. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. August 21,. When drawing a lewis dot structure, we are always attempting to achieve an electron count at which all of the atoms involved are stable and (usually) have full octets. In the structure of nacl each na + ion is surrounded by six chloride ions. Together, they form nacl, one unit of sodium chloride. Web sodium chloride, also known as salt. In the structure of nacl each na + ion is surrounded by six chloride ions. For the nacl lewis structure, calculate the total number of valence electrons for the nacl m.more. The structure of nacl is formed by repeating the face centered cubic unit cell. Schematic representation of valence electrons of a molecule in line (bonds) and dot (electrons) forms. Web lewis dot structure for sodium chloride. It is extracted from the mineral form halite or evaporation of seawater. Web an explanation of how you can accurately draw a diagram of the sodium chloride crystal structure The cell looks the same whether you start with anions or cations on the corners. Web rock salt (\(\ce{nacl}\)) is an ionic compound that occurs naturally as white crystals. Web crystal structure of sodium chloride. Schematic representation of valence electrons of a molecule in line (bonds) and dot (electrons) forms is lewis structure. Both ions show octahedral coordination (cn = 6). Here is how to draw the crystal structure of sodium chloride (nacl). All octahedral holes in a cubic close packing are occupied by counterions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g. Web nacl has a cubic unit cell. When drawing a lewis dot structure, we are always attempting to achieve an electron count at which all of the atoms involved are stable and (usually) have full octets. Since they’re from opposite sides of the periodic table, they form an ionic compound. Web nacl lewis structure & characteristics: In the structure of nacl each na + ion is surrounded by six chloride ions.Nacl structure,nacl crystal structure,how to draw nacl structure
Sodium Chloride Nacl Molecular Structure HighRes Vector Graphic
Structure of NaCl Easy trick to draw sodium chloride structure (Full
Chemical Bonds
How To Draw Sodium Chloride (NaCl) Diagram? YouTube
Formula unit of NaCl, sodium chloride Chemistry Dictionary
Class1112th। How to draw Nacl crystal structure? Nacl क्रिस्टल संरचना
Sodium Chloride Nacl Molecular Cube HighRes Vector Graphic Getty Images
How to draw NaCl (Sodium chloride) unit Cell? Solidstate Chemistry
Vector Ballandstick Model Of Chemical Substance Icon Of Sodium Chloride
Get All Your Chemistry Doubts Resolved By Enthuziastic Expert Teachers.
The Intramolecular Bonding Is Ionic, As It Involves The Transfer Of Electrons From Sodium To Chlorine, And Bonds Ions Through Electrostatic Forces.
101K Views 4 Years Ago Lewis.
Together, They Combine To Form A Unit Of Nacl.
Related Post: