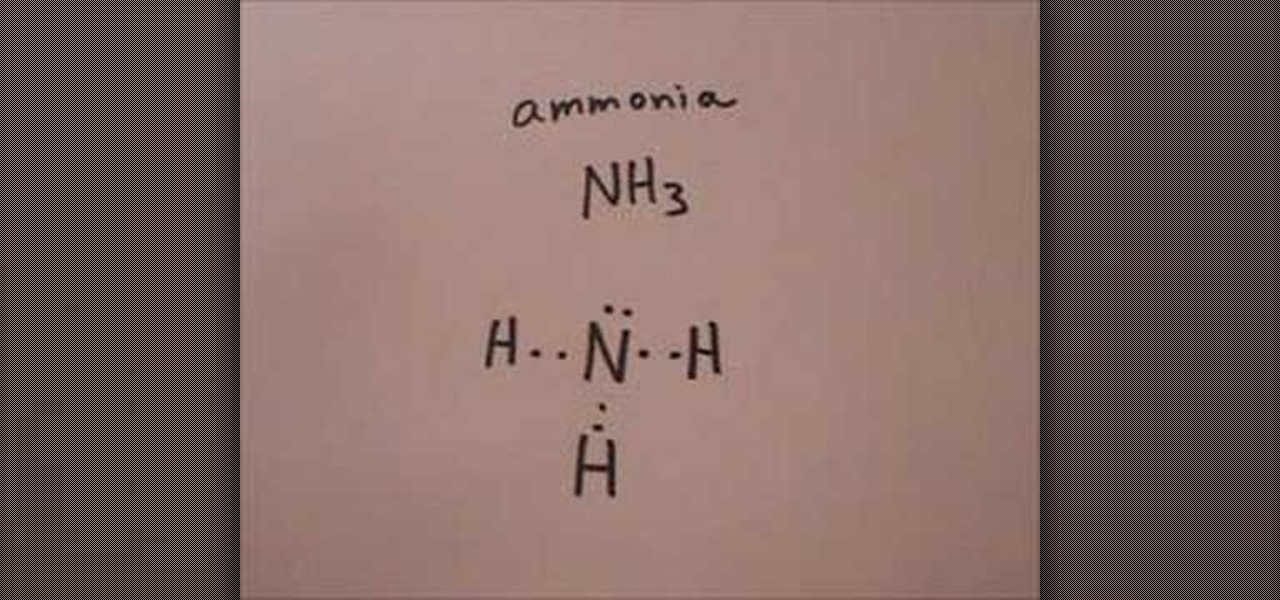Nh3 Drawing
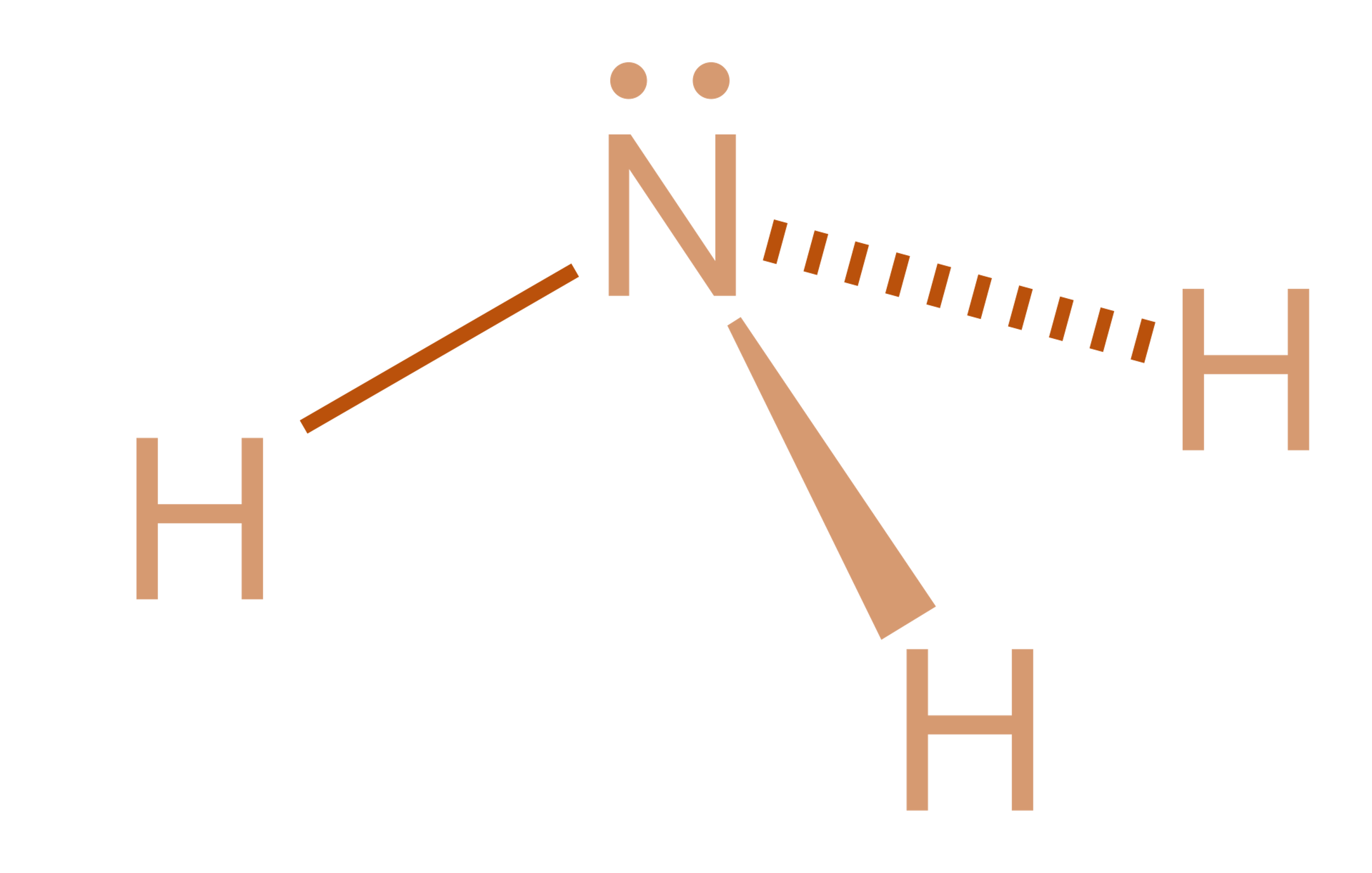
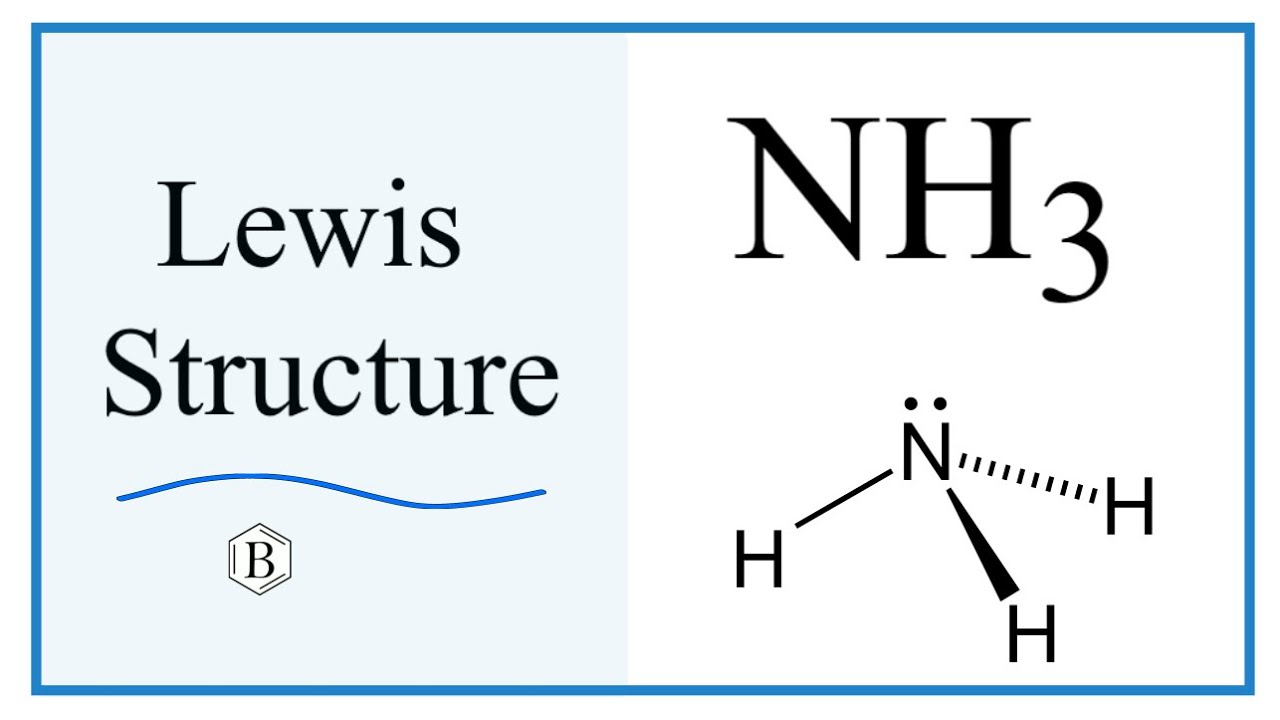
Nh3 Drawing - Calculate the total number of valence electrons. Nitrogen (n) is in group 15 of the periodic table, which means it has 5 valence electrons. Nh 3 (ammonia) is a commonly tested lewis structure. Web nh3 molecular geometry. This will help you understand the molecule’s electronic structure and bonding. There are 3 single bonds between the nitrogen atom (n) and each hydrogen atom (h). Understand how the dots represent the valence electrons and how they interact to form chemical bonds. Web here in this article, we discuss only the nh3 lewis dot structure, its hybridization, shape, and molecular fact in detail, and the nh3cl+ lewis dot structure. 159k views 4 years ago. Web learn about the dot diagram for nh3, including its lewis structure, electron arrangement, and molecular geometry. Web lewis structure of nh3. Here, we need to study how the lewis structure of the nh3 molecule is drawn: Web i quickly take you through how to draw the lewis structure of ammonia, nh3. These are arranged in a tetrahedral shape. Web a video explanation of how to draw the lewis dot structure for ammonia, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and bond angles. Remember to consider the octet rule, evaluate formal charges, and adjust the structure to minimize formal charges. All the hydrogen atoms are arranged symmetrically around the nitrogen atom which forms the base, and the two nonbonding electrons form the tip which makes the molecular geometry of nh3 trigonal pyramidal. It also is a good example of a molecule with a trigonal prymidal molecular geometry. For resonance structures there must be a double or triple bond present, which is not the case with nh3. There are 8 valence electrons available for the lewis structure for nh 3. Ammonia has a tetrahedral molecular geometry. In its aqueous form, it is called ammonium hydroxide. Web ammonia is a colorless gas with a chemical formula nh 3. Web lewis structure of nh3. Calculate the total number of valence electrons. Here, we need to study how the lewis structure of the nh3 molecule is drawn: Drawing the lewis structure for nh3. Web nh3 (ammonia) lewis structure has a nitrogen atom (n) at the center which is surrounded by three hydrogen atoms (h). I also go over hybridization and bond angle. In the nh3 lewis dot structure, n formed three sigma bonds with three h atoms. In its concentrated form, it is dangerous and caustic. They follow the duet rule (2 electrons). Remember to consider the octet rule, evaluate formal charges, and adjust the structure to minimize formal charges. Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). Web nh3 molecular geometry. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Web i quickly take you through how to draw the lewis structure of ammonia, nh3. Steps of drawing the lewis structure of nh3 is explained in detail in this tutorial. Web this nh3 lewis structure depicts the central nitrogen atom bonded to three hydrogen atoms. These are. Web drawing the lewis structure for nh 3. This inorganic compound has a pungent smell. For resonance structures there must be a double or triple bond present, which is not the case with nh3. Ammonia is a colorless gas with a distinct odor. They follow the duet rule (2 electrons). Nh 3 (ammonia) is a commonly tested lewis structure. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Web this nh3 lewis structure depicts the central nitrogen atom bonded to three hydrogen atoms. I also go over hybridization and bond angle. There is 1 lone pair on the nitrogen atom (n). Remember to consider the octet rule, evaluate formal charges, and adjust the structure to minimize formal charges. Nh 3 (ammonia) is a commonly tested lewis structure. Web lewis structure of nh3. Here, we need to study how the lewis structure of the nh3 molecule is drawn: Web here in this article, we discuss only the nh3 lewis dot structure, its. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Web ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture as a fertilizer. In its aqueous form, it is called ammonium hydroxide. Web 6 steps to draw the lewis structure of nh3 step #1: It's not particularly. Nh 3 (ammonia) is a commonly tested lewis structure. Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). In the nh 3 lewis structure (and all structures), hydrogen goes on the outside. Web lewis dot of ammonia. Web there is really only one way to draw the lewis structure for ammonia. The nh 3 chemical name is ammonia. There are 8 valence electrons available for the lewis structure for nh 3. Web nh3 (ammonia) lewis structure has a nitrogen atom (n) at the center which is surrounded by three hydrogen atoms (h). Join us as we delve into the intricacies of ammonia's molecular structure and learn how to draw its lewis. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. Web ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. Web nh3 molecular geometry. Web a. For resonance structures there must be a double or triple bond present, which is not the case with nh3. Web ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture as a fertilizer. Ammonia is commonly found in nature and is produced through the decay of. Lewis structure of nh3 can be drawn by. Nh 3 (ammonia) is a commonly tested lewis structure. Ammonia has a tetrahedral molecular geometry. Web 6 steps to draw the lewis structure of nh3 step #1: Web lewis dot of ammonia. Here, the given molecule is nh3 (ammonia). Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. It also is a good example of a molecule with a trigonal prymidal molecular geometry. Web nh3 (ammonia) lewis structure has a nitrogen atom (n) at the center which is surrounded by three hydrogen atoms (h). I also go over hybridization and bond angle. In order to draw the lewis structure of nh3, first of all you have to find the total number of valence electrons present in the nh3 molecule. Web nh3 molecular geometry. Web in this video i draw the dot and cross diagram for nh3 (ammonia). Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). 159k views 4 years ago. Join us as we delve into the intricacies of ammonia's molecular structure and learn how to draw its lewis structure. There is 1 lone pair on the nitrogen atom (n).Nh3 Estrutura De Lewis
Ammonia (NH3) molecule. Stylized skeletal formula (chemical structure
Nh3 Lewis Structure Molecular Geometry
Nh3 Molecule Structure
How to Draw the Lewis structure for ammonia « Science Experiments
NH3 Molecular Geometry Science Education and Tutorials
The Nh3 Lewis Dot Structure Understanding The Basics vrogue.co
Molecular Model Of Ammonia Nh3 Molecule Vector Illust vrogue.co
Electron Geometry for NH3 (Ammonia) YouTube
Draw The Lewis Structure Of Ammonia Nh3 Drawing.rjuuc.edu.np
Here, We Need To Study How The Lewis Structure Of The Nh3 Molecule Is Drawn:
In The Nh 3 Lewis Structure (And All Structures), Hydrogen Goes On The Outside.
This Will Help You Understand The Molecule’s Electronic Structure And Bonding.
For Resonance Structures There Must Be A Double Or Triple Bond Present, Which Is Not The Case With Nh3.
Related Post: